Research

REGULATION OF NEUTROPHIL GENE EXPRESSION IN INFLAMMATORY DISEASE & AGEING
The major theme of my research is investigating the role of neutrophils in the pathophysiology of inflammatory diseases such as rheumatoid arthritis (RA) and in ageing. Research projects are focussed on understanding the regulation of neutrophil gene expression, identification of neutrophil-derived molecular biomarkers of response to therapy, discovery of novel targets for the development of new therapies, and understanding the molecular effects of therapeutics (anti-TNF therapy, anti-IL17A therapy, JAK inhibitors) on neutrophil function in inflammatory disease. My research combines functional laboratory assays on freshly-isolated peripheral blood neutrophils from patients and healthy controls stimulated in vitro with relevant agonists, and RNA-Seq to understand the phenotype of inflammatory neutrophils. As an Arthritis Research UK Foundation Fellow at the University of Liverpool I made a significant contribution to the understanding of neutrophil gene expression during inflammation, including publishing the first RNA-seq analysis of primary human neutrophils, cells which are notoriously difficult to work with in vitro, including making two datasets available to the public (GSE40548, GSE70068). I also published the first analysis of neutrophils from RA patients using RNA-Seq, identifying signalling pathways that correlate with disease activity. My current Career Development Fellowship has allowed me to extend our understanding of neutrophil gene expression in rheumatoid arthritis using computational modelling. Recent funding has allowed me to extend my molecular study of RA neutrophils using quantitative proteomics (Wellcome Trust Seed Award) and 1H NMR metabolomics (Pfizer Aspire grant).
Current funding: Lupus UK
Previous funding: Career Development Fellowship Versus Arthritis (personal fellowship), Illix R&D Ltd, Wellcome Trust ISSF.
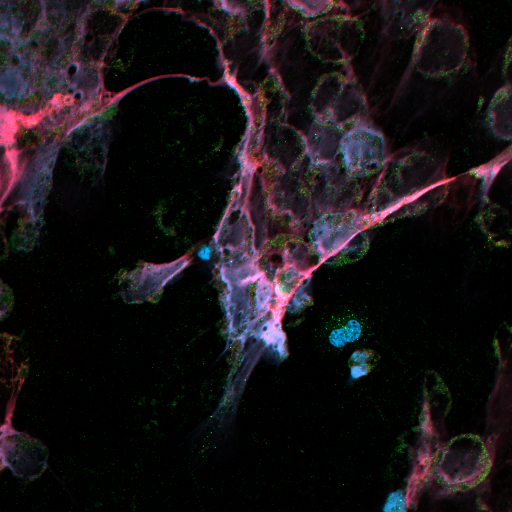
REGULATION OF NEUTROPHIL EXTRACELLULAR TRAP FORMATION
Neutrophil extracellular traps (NETs) kill pathogens via the release of chromatin and anti-microbial granule enzymes in an extracellular mesh or “net”. NET release represents a novel form of cell death (NETosis), distinct from apoptosis and necrosis, that can be induced by inflammatory agents (LPS, TNFα, IFNα) and micro-organisms. During NETosis, ROS production and protein-arginine deiminase (PAD)-4 activation induces histone citrullination, chromatin disruption and disintegration of the nuclear membrane. Granule enzymes are released from membrane-bound organelles into the cytoplasm, whereupon they mix with histones and decondensed chromatin, before the protein-loaded DNA is released via disruptions in the cell membrane. NETs may also a source of autoantigens in autoimmune diseases such as systemic lupus erythematosus (SLE) where they contribute to organ damage and nephritis. Emerging evidence also implicates NETs in RA as a source of citrullinated neoepitopes that can lead to loss of immune tolerance and development of antibodies to citrullinated proteins (ACPA), a hallmark of severe RA. This new evidence creates new avenues for understanding the role of neutrophils in RA. Serum autoantibody profiles in SLE and RA are different: the former typically have high titres of anti-dsDNA and anti-nuclear protein antibodies, while the latter have high ACPA. This suggests that either the molecular properties of NETs produced in SLE and RA are different and expose distinct autoantigens, or the host immune response to NETs in SLE and RA is different. This project is combining quantitative proteomics and immune-fluorescent staining to determine the molecular properties of NETs in RA and SLE.
Current projects: Self-funded PhD studentship (Mrs Sarah Shirley, with Dr Jill Madine ISMIB),
Previous funding: Connect Immune Research partnership with the Lorna & Yuti Chernajovsky Biomedical Research Foundation (with Prof Pat Eyers).
NEUTROPHIL METABOLOMICS IN INFLAMMATION AND AGEING
Neutrophils are phagocytic innate immune cells that play essential roles in host defence, but are also implicated in inflammatory diseases such as rheumatoid arthritis (RA) where they contribute to systemic inflammation and joint damage. Transcriptomic analysis of neutrophils has revealed significant changes in gene expression in neutrophils activated in vitro by cytokines and in vivo during inflammation in RA. However, there are no reports on the global metabolomic changes that occur during neutrophil activation. We already know that cellular metabolism is key regulator of neutrophil energy production, activation and function under conditions of both homeostasis and inflammation. Fine-tuning of metabolism during an inflammatory response is key for the generation of small molecule metabolites such as ATP, NADPH, nucleotides and amino acids, which are required rapidly and in high abundance during cellular activation. Neutrophils rely heavily on glucose metabolism via glycolysis to fuel their energy requirements. The first intermediate of glycolysis, glucose 6-phosphate (G6P), fuels the pentose phosphate pathway (PPP), where NAPDH is produced to fuel NOX2 activity, ROS production, and NOX2-dependent NET formation. We are using 1H NMR spectrometry to investigate the intracellular changes in the neutrophil metabolome during in vitro and in vivo inflammation. In particular, we are interested in the changes to the metabolome which take place in RA patients during treatment with therapies such as JAK inhibitors.
Current funding: PhD scholarship on joint program with Chulalongkorn University and the University of Liverpool (Dr Grace Filbertine), Dunhill Medical Trust (Miss Genna Abdullah, PhD studentship).
Previous funding: PhD scholarship from Versus Arthritis and the Masonic Charitable Foundation
All projects with Dr Marie Phelan.
Research grants
Proteomic analysis of blood serum in healthy ageing and frailty
VIVENSA FOUNDATION (UK)
February 2025 - August 2025
Investigating the role of small non-coding RNAs in regulating gene expression and activation in SLE neutrophils
LUPUS UK (UK)
April 2025 - March 2026
Defining the pro-reparative roles of neutrophils in fracture responses: relationship to inflammaging and frailty
VIVENSA FOUNDATION (UK)
April 2024 - April 2027
Neutrophil extracellular traps (NETs) as a therapeutic target for the treatment of auto-immune inflammatory disease
VERSUS ARTHRITIS (UK)
September 2022 - November 2023
Computational modelling and functional investigation of neutrophil activation and plasticity in inflammatory disease
ARTHRITIS RESEARCH UK
June 2017 - March 2024
Evaluation of novel mechanisms for inhibiting rheumatoid arthritis
AINTREE ARTHRITIS TRUST (UK)
October 2017 - September 2024
Biomarkers in inflammation
ILLIX LIMITED (UK)
May 2018 - November 2020
Multi-modal high resolution preclinical PET+SPECT+CT scanner
WELLCOME TRUST (UK)
July 2018 - June 2024
The effect of JAK inhibition on neutrophil killing, NETosis and metabolism in rheumatoid arthritis
PFIZER LTD (UK)
October 2016 - July 2017
Molecular properties of neutrophil extracellular traps (NETs) in rheumatoid arthritis (RA)
WELLCOME TRUST (UK)
June 2016 - May 2018
Secukinumab, neutrophils and vitamin D in Psoriatic Arthritis
NOVARTIS PHARMACEUTICALS UK LTD (UK)
May 2015 - December 2019
Role of neutrophils in inflammation induced liver cancer following liver fluke infection
DEPARTMENT FOR BUSINESS, ENERGY AND INDUSTRIAL STRATEGY (BEIS) (UK)
April 2016 - June 2017
Bridging and accelerating the translation of novel scientific findings for health and wealth gain
MEDICAL RESEARCH COUNCIL
March 2014 - August 2015
Changes in neutrophil gene expression during active and resolved inflammation
ARTHRITIS RESEARCH UK
December 2010 - April 2014
Research collaborations
Dr Zoe McLaren
Clinical collaboration at LUHFT
Liverpool University Hospital Foundation Trust
Clinical collaboration in rheumatology
Prof Chrissy Hammond
Models of frailty and aging
Univeristy of Bristol
CoI on Dunhill Medical Trust project led by Bristol team to investigate inflammation and frailty fractures
Dr Marie Phelan
Neutrophil metabolomics
NMR Metabolomics
Dr Asan Akpan
Neutrophils in frailty
University of Western Australia
Supervision of Miss Genna Abdullah (PhD student)
Prof Roy Goodacre
Metabolomics (IR / Raman)
IR / Raman analysis of neutrophil metabolites
Dr Jill Madine
Inflammation in aortic dissection
Joint supervision of PhD student Mrs Sarah Shirley
Dr Direkrit Chiewchengchol
Neutrophils metabolomics in SLE
Chulalongkorn University
Joint supervision of PhD student Dr Grace Filbertine
Dr Borko Amulic
Models of frailty and aging
University of Bristol
CoI on Dunhill Medical Trust project led by Bristol team to investigate inflammation and frailty fractures