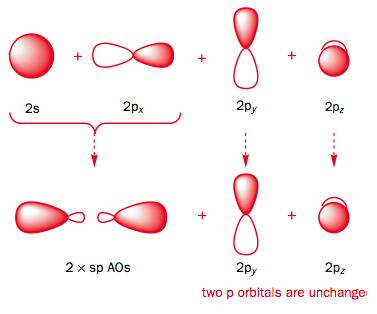
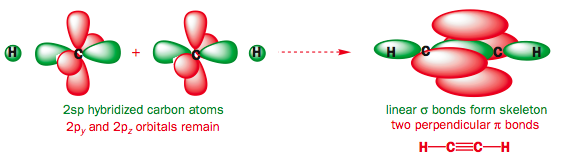
Use the buttons to display the Hydrogen 1s and Carbon sp orbitals that make up the sigma framework and the remaining p orbitals which form the two pi-bonds.
Observe that the two pi orbitals are perpendicular.
|
Hydrogen | Fluorine | Methane | Ethylene | Acetylene | Allene | Formaldehyde | Benzene | p-orbitals | d-orbitals | f-orbitals