Red regions are electron rich (negative) and blue regions are electron depleted (positive). Note where the red regions are in each of these amines. Are the amine lones pairs available to act as bases?
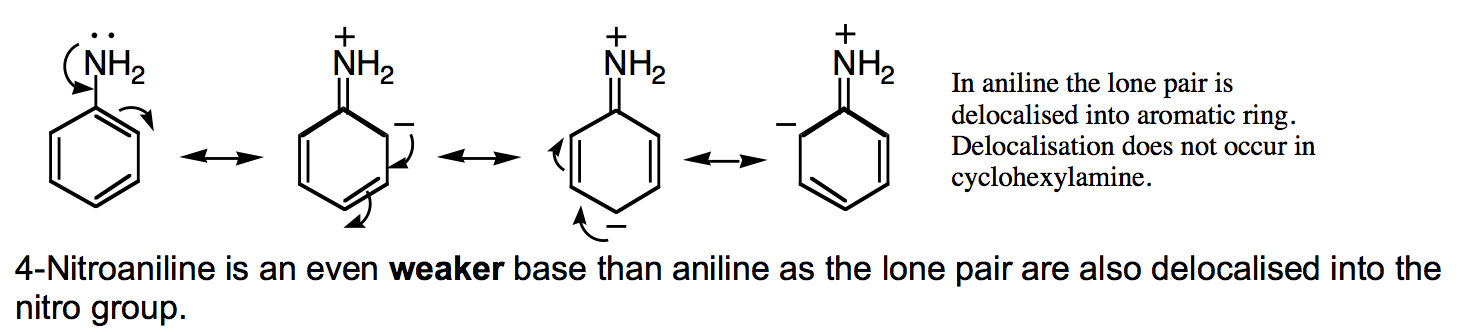
Note the progressive flattening of the NH2 group.
Cyclohexylamine |
Aniline |
4-Nitroaniline |

Hydrogen | Fluorine | Methane | Ethylene | Acetylene | Allene | Benzene | p-orbitals | d-orbitals | f-orbitals