Electrophoresis of haemoglobin - use in diagnosis: Theory
6.1 Introduction
The aims of this practical are:
6.2 Theory and background
Sickle cell diseaseSickle cell disease is caused by a hereditary defect in the haemoglobin molecule. The two b chains in normal haemoglobin (Hb-A) contain a glutamic acid residue at position 6. In people with sickle cell disease, a valine residue occurs in this position due to an A to T transversion mutation in the glutamate codon GAG to give the valine codon GTG. This residue is on the outer surface of the molecule and this single difference in the sequence of the 146 amino acids of the b chain is enough to produce a "sticky" hydrophobic spot on the surface that results in the abnormal quaternary association of the a and b chains of the abnormal haemoglobin (Hb-S).
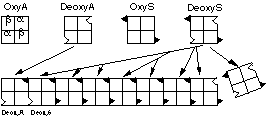
Both deoxygenated Hb-A and Hb-S have a normal, "complementary" sticky patch on the surface of the a chains to which the abnormal hydrophobic spot on deoxygenated Hb-S will bind. This normal patch is masked when the haemoglobin is oxygenated. Thus, when oxygen concentrations fall below a critical level, the Hb-S polymerises into linear, insoluble arrays of fibres within the erythrocyte, which become deformed (sickled) and function abnormally (Figure 1). This only happens in sickle cell homozygotes, since the presence of deoxyHb-A produced by the normal allele in heterozygotes will terminate the polymerisation. Heterozygotes are phenotypically normal but are said to carry the "sickle cell trait"

Figure 1: Polymerisation of Hb-S
The Glu to Val substitution also causes a charge difference between Hb-A and Hb-S that affects the mobility of the molecule in an electric field. Thus, electrophoresis of haemoglobin (or a red cell lysate – haemolysate , which is predominantly haemoglobin) can be used as a diagnostic aid and can readily distinguish between normal, sickle cell homozygote and sickle cell heterozygote individuals.
Electrophoretic separation encompasses a variety of methods in which solutes (usually charged macromolecules such as proteins, nucleic acids etc.) are resolved on the basis of their mobility in an electric field. In general, an electric field is applied through some supporting medium and the solutes migrate in that field, according to:
The supporting medium may be one of several types:
Methods of separation and apparatus vary widely, but in general terms a sample is applied to the support in a buffer that allows maximum separation of differently charged solutes – this can be highly dependent upon pH and ionic strength. An electric field is applied and the different components will move in the support, negatively charged molecules towards the anode and positively charged ones towards the cathode (Figure 2).
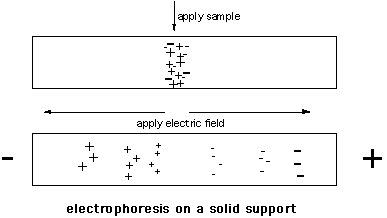
Figure 2: Electrophoresis on a cellulose acetate strip