PRACTICAL 6
Electrophoresis of haemoglobin - use in diagnosis: Methods
6.3 Experiment A: Separation of Haemoglobins A and S by electrophoresis on cellulose acetate strips
.
Reagents:
Haemolysates A, B and C (a haemolysate is the contents of the red cells). You will be given a 5 Ál sample of each by your demonstrator.
Electrophoresis buffer (TEB – 0.12 M Tris, 5 mM EDTA, 15 mM boric acid, pH 8.9)
Protein stain solution (0.5% Ponceau S in 5% TCA [trichloroacetic acid]). N.B. TCA is corrosive. Take great care with this solution. WEAR GLOVES.
Destain solution (5% acetic acid)
Procedure (work in pairs for this experiment)
N.B. Steps 1 and 2 will probably have been done for you. If so, proceed to step 3.
- Place TEB electrophoresis buffer (about 500 ml total) into all four compartments of the electrophoresis tank. Ensure that the level is the same in all compartments by carefully lifting the tank so that the buffer laps over the end of the separating walls. Wipe any excess liquid from the walls with a tissue.
- Thoroughly wet 2 pieces of Whatman no. 3 filter paper (20 x 7.5 cm) with buffer solution and place them over the edges of the shoulder pieces with one edge of the paper running parallel with edge of the shoulder and the other immersed in the buffer of the outer compartment. These pads act as buffer wicks between the buffer solution and the cellulose acetate strips which are placed between them.
- Take one cellulose acetate strip and, with a blunt pencil (provided) , write your initials at one end. Also mark a faint starting line (origin) lightly across the centre of the strip – see Figure 6. (Note: handle strips at the ends only or use the plastic forceps). Moisten the strip as follows. Add some TEB buffer to a shallow dish and carefully float the strip on top so that it is impregnated with buffer from below by capillary action. When the strip is thoroughly wetted (3-4 min), it can then be submerged in the buffer. It is essential that this procedure is followed exactly since it avoids trapping air bubbles in the pores of the membrane.
- Remove excess buffer from the strip by blotting lightly on filter paper – do not overdry by pressing too hard.
- You will be given a 5 Ál droplet of each of the three haemolysates (A, B and C) on a small square of Parafilm by your demonstrator. Apply the sample applicator to the surface of droplet A then transfer the sample A to the left hand one-third of the pencilled origin line on the strip by gently touching the applicator on to the strip (see Figure 6 ). Rinse the applicator with distilled water from a wash bottle, dry, then repeat the process for haemolysates B and C (rinsing between each). Apply B to the centre one-third of the origin line and C to the right hand one-third (see Figure 6).
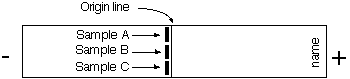
Figure 6: Sample application on celluose acetate strip
- Place the strip between the two shoulder pads of the electrophoresis tank, with the origin in the centre and the end with your name towards the anode and carefully pull taut. Press the ends of the strip firmly against the pad to ensure proper contact.
- Place the Perspex lid on the tank and connect the red and black terminals (male) of the tank to the +ve and -ve terminals (female) of the power supply. Ask a demonstrator to check the correct set-up and the demonstrator will switch on the power supply. Electrophoresis will be carried out at a constant voltage of 150 V (approx. 0.5 mA/cm strip width) for 60 mins.
- Switch off and unplug the power supply. Remove the strip with forceps and carefully float it on the surface of the Ponceau S staining solution, allowing the stain to impregnate the strip from below. When totally wetted, immerse the strip completely in the stain and leave for 5 mins. Agitate occasionally.
- To destain the strip, remove it from the stain. Drain off excess stain and rinse in a tray of 5% acetic acid. Change the acetic acid once, agitate for a moment, then finally rinse the strip in tap water.
Diagnosis
Given the nature of the amino acid substitution and the direction in which the protein bands have moved, which of the three samples, A, B and C, represent normal, sickle cell heterozygous and sickle cell homozygous individuals? Why?
Return to  .Practicals Home Page......
Return to
.Practicals Home Page......
Return to  .Practical 6: Main page......
Return to
.Practical 6: Main page......
Return to  .Practical 6: Theory
.Practical 6: Theory

