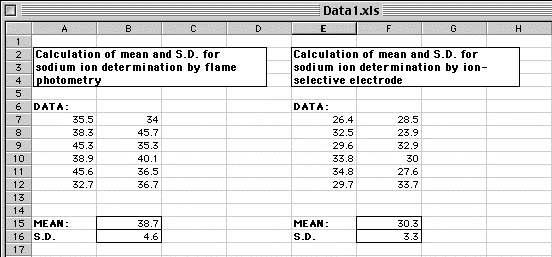
Determination of sodium ion concentrations: Class Data
The class data for the determination of sodium by flame photometry and ion-selective electrode are provided here for simple statistical analysis (mean and standard deviation) using an Excel spreadsheet.
A template Excel spreadsheet has already been prepared and you can download it by clicking here. Save a copy on your hard disk then open it in Excel either by double clicking it, or from the File menu if Excel is already running (do not try to open it directly then modify it or you may experience problems, depending on the browser and system set up you are using).
All concentrations are in mM.
Class 1 (First Friday, a.m.), October 14th 2011
NOTE: The extra calculated data have not been e-mailed to me (black mark to those who promised) so you will just have to go ahead with the data we have.|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|
|
[Na+](flame) |
47.5 |
40.0 |
60.0 |
61.7 |
47.5 |
65.0 |
22.5 |
100 |
67.5 |
25.0 |
17.5 |
66.3 |
|
[Na+] (ISE) |
57.5 |
47.8 |
70.8 |
59.3 |
70.8 |
46.8 |
69.2 |
25.7 |
49.0 |
60.3 |
- |
- |
Class 2 (Second Friday, a.m.), October 21st 2011 No class this year
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|
|
[Na+](flame) |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
[Na+] (ISE) |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
.
CONCLUSIONS:
Using all of the submitted flame photometer results gives a sodium ion concentration of 51.7±23.4 mM.
Omitting the 4 values that seem to be way out improves the data to 56.9±10.4 mM. Similarly, using all the ISE data
gives 55.7±14.0 mM while omitting the one poor result gives 59.1±9.8 mM. The true, calculated value was 60 mM so
the mean ISE result is very close. The FP result is not bad either. In terms of
what was predicted, the ISE is indeed higher than the FP, which is consistent with its overestimation of sodium
due to a partial response to potassium. However, there is no significant difference in SD, which is a measure
of reproducibility. The ISE was predicted to give a smaller SD. However, both SDs are very high due to differences
in the way different students carried out the experiment.
