Research
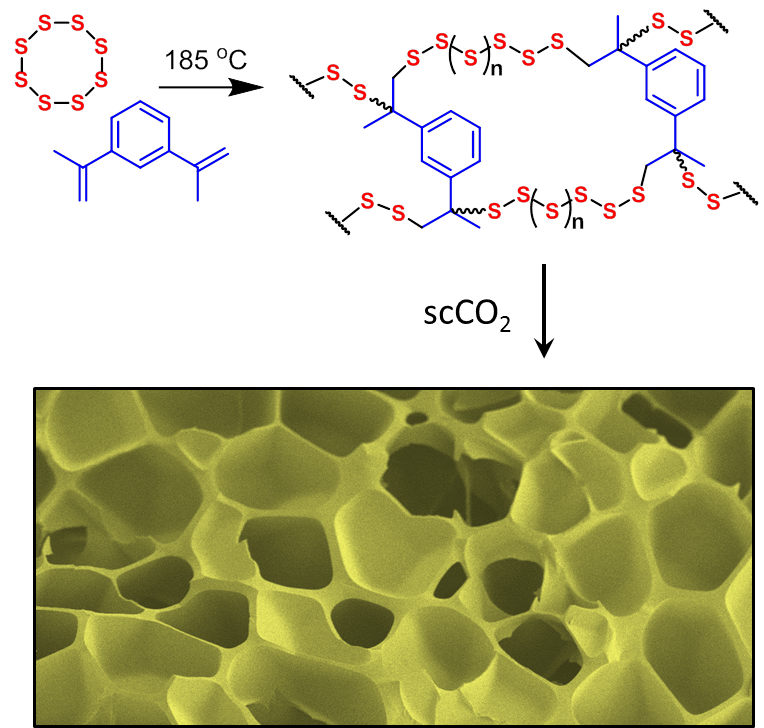
Functional porous materials from inorganic waste
Porous materials are permeable, high surface area materials with applications in gas storage, catalysis, and filtration. There has been considerable interest in porous materials over the last ten years, and metal-organic frameworks and porous polymers with incredible properties have been reported. However, many of these new materials are limited in application due to the high cost of production. We are developing new porous materials from inorganic waste and other low cost or renewable resources. The target is to produce materials with superior properties, but at a cost that makes them useful for widespread practical applications, especially filtration of toxic pollutants from water and air flows. A good example is sulphur-polymers. Sulfur is an industrial by-product of oil refining. We recently showed that when polymers made from elemental sulfur are made porous, they can be used to filter mercury from water.
- Porous inverse vulcanised polymers for mercury capture
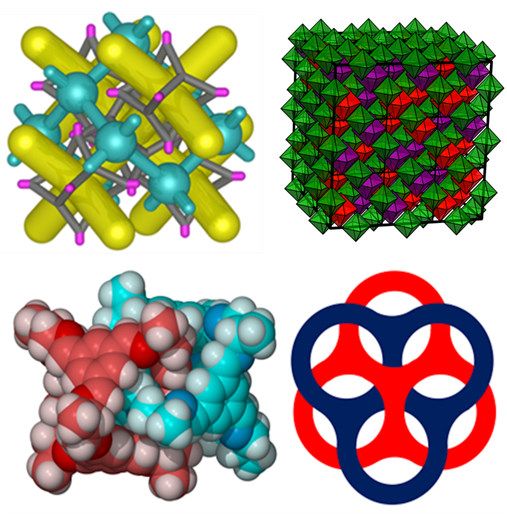
Porous organic cages
Much of Tom's research in Liverpool has been on the synthesis and characterisation of porous organic cages and their applications. Cages are a relatively new area of porous materials. Unlike extended frameworks, they are discrete, soluble molecules. The porosity is prefabricated and intrinsic to the molecular cage structures - which contain an internal void and open window to allow guest access.
Key papers:
- Cages for SF6 separation
- Directing the crystallisation of cages - controlling polymorphs
- Three component cage cocrystals - Porous organic alloys
- Cage nanocrystals
- Catenated cages - Triply interlocked molecules
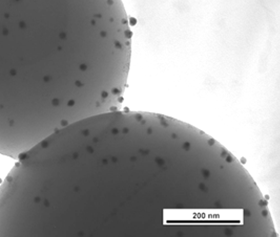
Supercritical carbon dioxide
Supercritical CO2, (scCO2) is a "green" solvent. It is produced when CO2 is heated above its critical temperature (32 degrees C) and compressed over its critical pressure (7.4 MPa). It then has liquid and gas like properties - like a gas it occupied the entire volume of the vessel, with no meniscus or surface tension, but like a liquid the molecules are close enough together to act as a solvent. It has tune-able density, high diffusivity, is non-combustible and non-toxic as well as being relatively environmentally benign, and as it is a gas at ambient temperatures and pressures it can be easily removed after reaction, leaving no solvent residues. It can be used in the processing and impregnation of organic polymers with inorganic components to form hybrid materials. Key papers:
- Silver Nanoparticle Impregnated Polycarbonate Substrates for Surface Enhanced Raman Spectroscopy
- Silver nanoparticle–polymer composites by supercritical CO2 polymerisation
- Palladium Nanoparticle Incorporation in CMPs
- Metal-polymer nanocomposites by supercritical fluid processing (book chapter)
Research groups
Research grants
Post-Consumer Resin - Understanding the quality-performance linkage for packaging
UK RESEARCH AND INNOVATION
November 2020 - June 2024
Unusual trisulfide chemistry
AUSTRALIAN RESEARCH COUNCIL (AUSTRALIA)
January 2023 - December 2025
Transition to a circular economy for plastics through inclusion of Post Consumer Recycled (PCR) plastic in household cleaning products packaging (including strong formulations like bleach)
WASTE AND RESOURCES ACTION PROGRAMME (UK)
September 2019 - December 2020
Enhancing the porosity of sulfur-polymers: a material made from waste than can clean up mercury pollution
ROYAL SOCIETY (CHARITABLE)
March 2018 - February 2022
Carbonised sulfur polymers for gold extraction
ROYAL SOCIETY
December 2017 - September 2025
High pressure equipment for the supercritical foaming of polymers, and drying of aerogels, for air and water filtration applications.
ROYAL SOCIETY
November 2017 - October 2018
High-sulfur polymers as matrices for heterogeneous catalysis
ROYAL SOCIETY (CHARITABLE)
March 2018 - March 2022
Second generation inverse-vulcanised sulfur polymers for mercury filtration
ROYAL SOCIETY
December 2016 - June 2018
Enhancement of the mechanical properties of sulfur based polymers, and investigation of their behaviour in supercritical carbon dioxide
ROYAL SOCIETY
March 2016 - September 2021