Research
Our research aims to understand the concepts and mechanisms that underlie the interface of advanced materials for healthcare and complex medicines with the immune system, to support their translation for clinical use, and to gain insight into the fundamental biology behind them. We use advanced techniques and technologies to explore and investigate these areas of research, as well as develop new techniques to address gaps in the characterisation knowledge base. To date, we have worked with a wide variety of complex medicines, from lipidic nanoparticles to polymeric and from virosomes to exosomes, for the delivery of diagnostics, small molecules, biopharmaceuticals, biopolymers, and nucleic acids. Our work is supported by funds from bodies including, but not limited to, the European Commission, Wellcome Trust, NIH and Kidney Research UK.
In addition to our academic research, we also offer our services and consulting to the development of nanobiomaterials, advanced therapeutics, and complex medicines. This is via the Nanotherapeutics Hub, based in the Centre of Excellence for Long-acting Therapeutics (CELT), were we can offer support in areas of immunology (e.g., immune cell responses, mixed lymphocyte reactions, immune cell function), haematology (e.g., haemolysis, complement activation, platelet activation, coagulation), and cellular health (e.g., cytotoxicity, cell death pathways, bystander responses to cell death/indirect immune stimulation). Examples of our assay capabilities may be found here
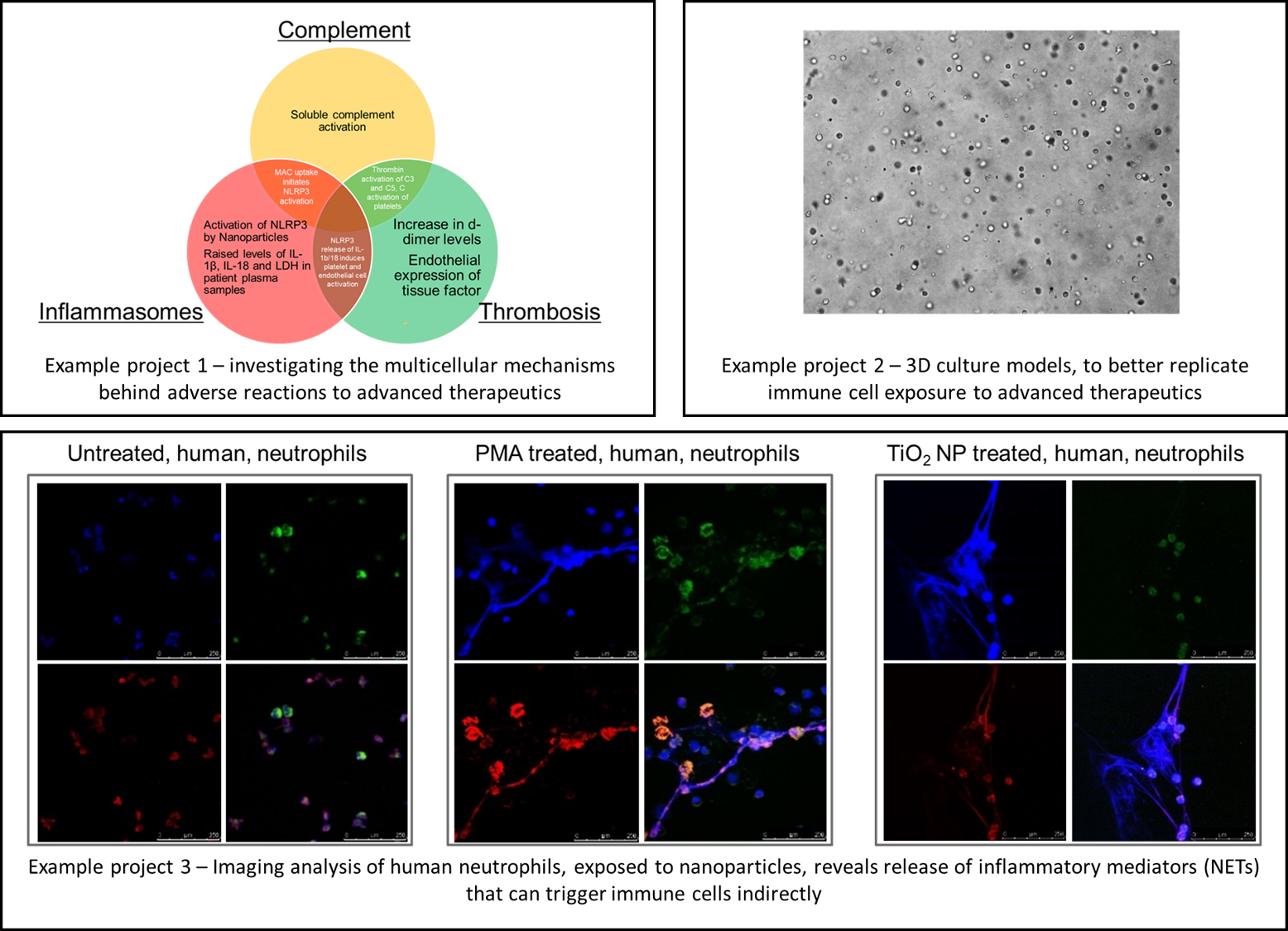
Advanced therapeutics, Biomaterials, and Complex Medicines: assessing their biocompatibility and toxicity.
Nanomaterial behaviour has been demonstrated to differ considerably from that of larger bulk materials. The production and use of engineered nanomaterials is constantly expanding, but there remain uncertainties surrounding the potential risks posed to human health and the environment. The size of nanomaterials influences their toxicity, and this has been consistently demonstrated for nanomaterials encountered in workplace environments (e.g. carbon black, polystyrene, titanium dioxide, and silver). Nanomaterials vary considerably with respect to their composition, charge, surface area, solubility, crystal structure, surface chemistry, and shape.
Many nanomaterials have been shown to interact with the immune system by either stimulating or suppressing various responses through their interaction with proteins found within the blood. Many nanomaterials interact with serum proteins such as opsonins (e.g. antibodies, complement) and are rapidly taken up into cells of the mononuclear phagocyte system (e.g. macrophages). Interactions with serum proteins may render nanomaterials antigenic and it has been suggested that functionalisation (e.g., with growth factors or receptors) may induce neutralising antibodies that also recognise the body’s own molecules, with implications similar to those for biotechnology-derived therapeutics. We are utilising a number of approaches to understand how nanomaterials interact with biological systems. Identification of these mechanisms may allow rational design of future materials and reduction of unwanted effects, particularly how complex systems can interact, to bring about clinical events, and how inflammation is key to the definition of Adverse Outcome Pathways (AOP)
Interaction of nanomaterials with the human immunological & haematological systems, pathways of cellular uptake of nanoparticles (both receptor and non-receptor mediated), nanotoxicity of nanomaterials, and interaction of nanoparticles with plasma proteins (biomolecular corona). immune modulation, hypersensitivity, and pseudo-allergies related to advanced materials such as liposomes and lipidic nanoparticles, liposomes, polymeric materials, inorganic nanoparticles, virosomes and exosomes, and nanobiomaterials.
Keywords: advanced therapies and therapeutics, liposomes, lipidic nanoparticles (LNP), CARPA, pseudoallergy, hypersensitivity, complex medicines, extracellular vesicles, nanomedicines, nanotherapeutics, biocompatibility, immunology, immunotoxicology, intracellular drug delivery (ICD)
Sub-project;
- Development of techniques and technologies for the characterisation of nanomaterials.
The field of nanoimmunotoxicology is constantly evolving as new materials are developed. In order to meet these challenges it is necessary to ensure that current techniques and methodologies are sufficient to identify nanomaterial interactions with biological systems that may be undesirable. New characterisation techniques are studied for their utility and introduced into our assay cascade as appropriate. Interaction with agencies such as EMA and FDA are also key in order to stay ahead of what is required from a regulatory perspective.
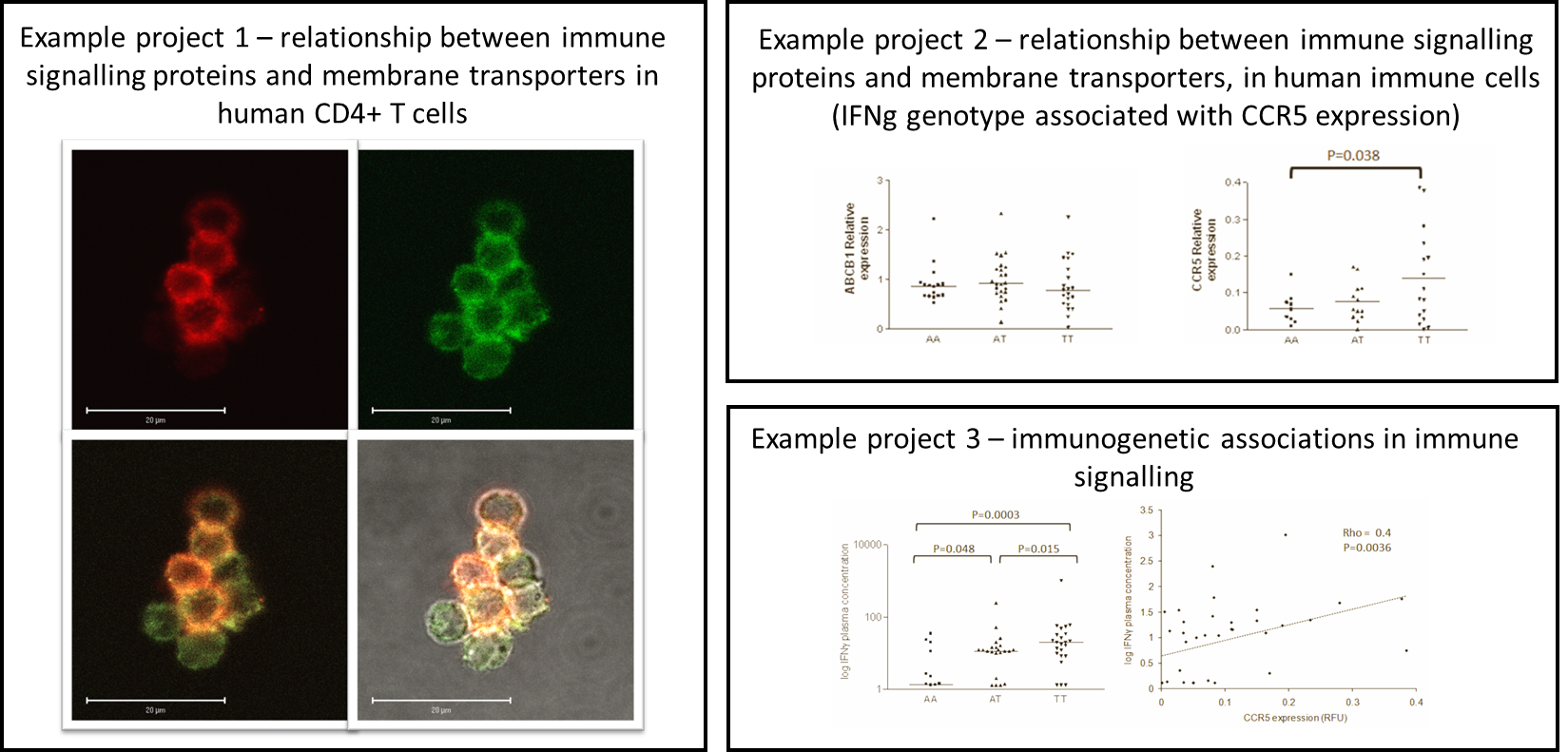
Impact of immune status on nanomaterial biocompatibility, drug disposition and intracellular pharmacology.
Dysregulation of the immune system is a hallmark of a number of diseases, such as HIV. The mechanisms by which the immune system can influence drug disposition are relatively understudied, but recent work has highlighted the necessity of examining its impact on pharmacokinetics and pharmacodynamics. A more comprehensive approach in clinical studies will allow better determination of the impact of cytokines on drug disposition. In addition, determining the mechanisms that underpin the differential effects of cytokines across different cell types will clarify the responses reported in these studies. This in turn relates to the biocompatibility of nanomaterials in patients with dysregulated immune systems as in HIV infection, specifically when comparing the response of uninfected vs infected cells.
Cytokines etc. are integral to the successful resolution of many diseases. Data are emerging on a role for regulation of the expression and activity of drug transporters and drug metabolising enzymes.. Investigation of the interaction between pharmacological and immunological responses is key to understanding the complex relationships involved in patient responses to therapy as well as how they may respond to nanoformulated therapies.
Keywords: immune response, drug disposition, human immune cells
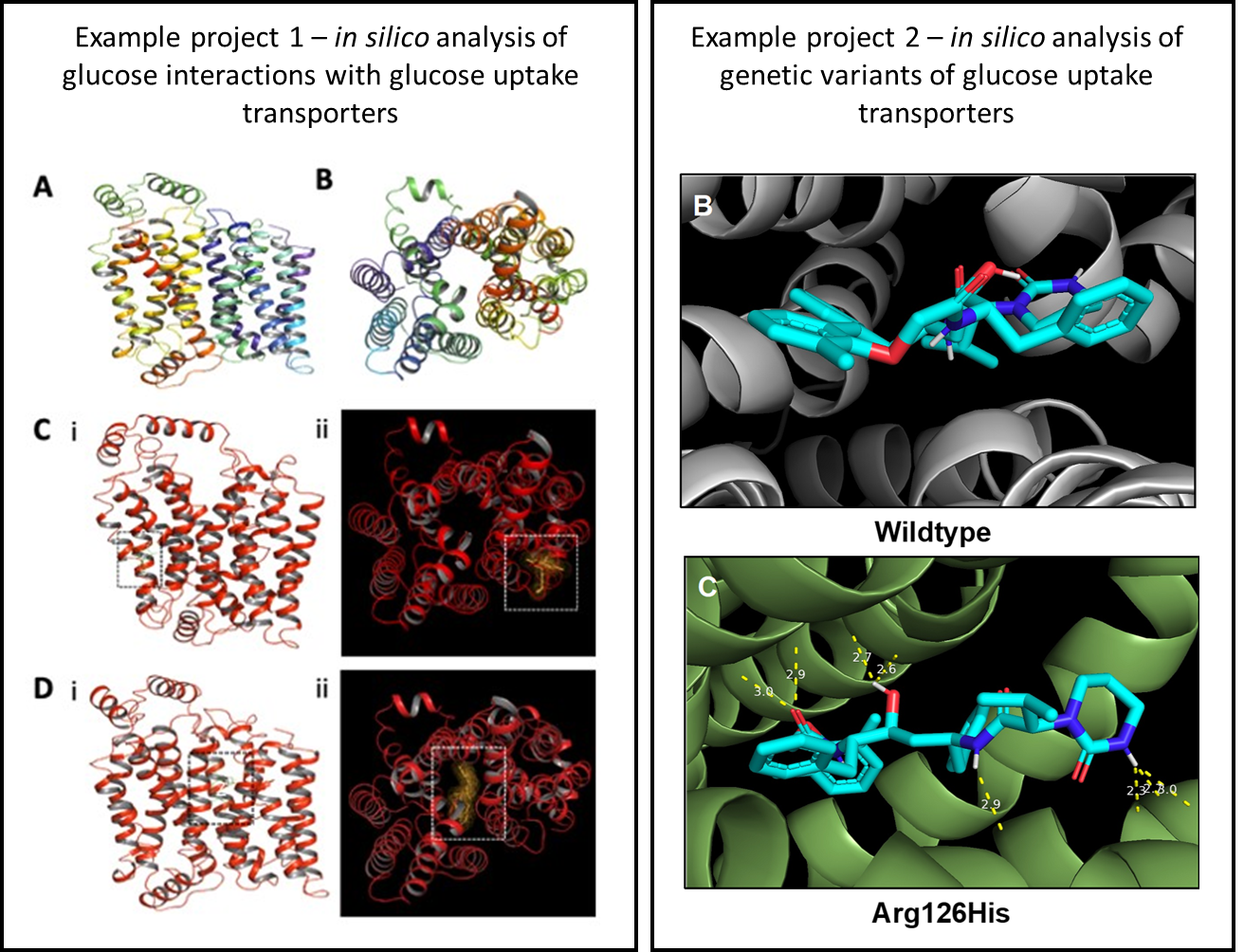
Influence of metabolic and bioenergetic profiles on innate immune modulation
Innate immune activation is tightly linked to metabolic reprogramming. When pattern recognition receptors, such as TLRs and RIG-I, detect danger signals, the NF-κB and IRF pathways activate cells to undergo high glycolysis. GLUT1 and GLUT4 rise, HIF-1α, PI3K, Akt, mTOR, and TBK1 drive hexokinase, PKM2, and LDH. This fuels rapid cytokine release, phagocytosis, and antimicrobial activity. This programme is helpful in acute infections, but persistent activation can harm tissues. In cancer, atherosclerosis, and neuroinflammation, sustained NLRP3 signalling and macrophage glycolysis contribute to the maintenance of pathological IL-1β, IL-6, and TNF-α. The same circuits can blunt the effectiveness of therapeutics by altering pharmacokinetics and triggering the formation of anti-drug antibodies, presenting opportunities to harness or temper these pathways to fit the indication.
Opportunities for modulation span metabolism and signalling. Glycolysis or lactate handling can be tuned, for example, by targeting enzymes such as hexokinase, PKM2, or LDH. AMPK activation or mTOR inhibition can rebalance overly glycolytic programmes. Inhibitors of NF-κB, JAK-STAT, or inflammasome assembly reduce the release of cytokines. PRR antagonists can prevent inappropriate sensing. Controlling transporter activity, such as GLUT1 and GLUT4, offers another lever. These strategies can boost host defence in infection while limiting collateral damage; they can also de-escalate chronic inflammation in non-communicable diseases and we have several research programs investigating this.
In addition, extracellular vesicles add a second lever. Tolerosomes and other regulatory EVs carry proteins and RNAs that dampen PRR signals and tune cytokine networks. They help maintain homeostasis and may explain inter-individual variation in responses. You can exploit EVs directly, or design nanovesicles that mimic their tolerogenic cues, to restrain NLRP3 activity, reduce IL-1β and IL-6, and preserve protective immunity and e have shown can impact on the innate immune response to several therapeutic modalities.
Innate immunity and metabolism are inseparable. By mapping the transporters, enzymes, and signals that couple PRR engagement to glycolysis and cytokine output, we are designing targeted interventions. These include small molecules, biologics, EVs, and nanomedicines that recalibrate innate responses for infection control and for chronic inflammatory conditions.
Keywords: innate immunity, immune metabolism, microenvironment, transporters
Research groups
Research grants
Biologics Regional Innovation and Technology Ecosystem (BRITE)
RESEARCH ENGLAND (UK)
July 2025 - June 2028
Lipid Voyage: Exploring the biology, safety and biotechnological application of lipid-phage complexes found in Breast Milk. (Lipid voyage)
BIOTECHNOLOGY & BIOLOGICAL SCIENCE RESEARCH COUNCIL
September 2024 - August 2027
Rational Engineering of Inorganic Crystals as Novel Therapeutics to Induce Antimicrobial Activity in Host Cells
INFECTION INNOVATION CONSORTIUM (UK)
November 2024 - June 2025
Building a UK IntraCellular Drug Delivery Centre (ICDDC)
INNOVATE UK (UK)
January 2023 - March 2026
A game changer for the treatment of osteoarthritis: a cost effective combined advanced therapy to treat knee osteoarthritis (SINPAIN)
UK RESEARCH AND INNOVATION
May 2022 - October 2026
National Hub for Advanced Long-acting Therapeutics (HALo)
ENGINEERING & PHYSICAL SCIENCES RESEARCH COUNCIL
November 2024 - October 2030
CF- TRAILFINDER (CF- TRAILFINDER)
CYSTIC FIBROSIS TRUST (UK)
December 2024 - November 2029
Automated high-resolution microscope platform with robotics to study pharmacodynamics in 3D cell cultures
MEDICAL RESEARCH COUNCIL
November 2022 - March 2023
Safety Testing In The Life Cycle Of Nanotechnology-Enabled Medical Technologies For Health (SAFE-N-MEDTECH)
EUROPEAN COMMISSION
April 2019 - September 2023
Regulatory Science Framework for Nano(bio)material-based Medical Products and Devices (REFINE)
EUROPEAN COMMISSION
December 2017 - February 2022
Assessing the therapeutic effects of renal regenerative medicine therapies using a suite of in vitro assays
CHILDRENS RESEARCH FUND (UK)
February 2021 - October 2021
European Nanotechnology Characterisation Laboratory
EUROPEAN COMMISSION
May 2015 - December 2019
Developing novel tools and technologies to assess the safety and efficacy of cell-based regenerative medicine therapies, focusing on kidney disease RenalToolBox
EUROPEAN COMMISSION
November 2018 - April 2023
Investigating how cell based regenerative medicine therapies modulate T-cells and macrophages to ameliorate acute kidney injury
KIDNEY RESEARCH UK (UK)
November 2018 - September 2021
Exploring how cell therapies ameliorate renal damage
MERSEY KIDNEY FIRST (UK)
October 2018 - September 2021
Long acting NRTI therapies for HIV
NATIONAL INSTITUTES OF HEALTH (USA)
July 2017 - June 2023
Multi-modal high resolution preclinical PET+SPECT+CT scanner
WELLCOME TRUST (UK)
July 2018 - June 2024
Bioengineered substrates for human pluripotent stem cell culture
WELLCOME TRUST (UK)
November 2016 - October 2018
Investigation of the use of nanotechnology to reduce the side effects associated with antiretroviral protease inhibitors
BRITISH SOCIETY FOR ANTIMICROBIAL CHEMOTHERAPY (UK)
May 2015 - December 2016
Characterising the influence of nanoparticle characteristics on their interference/ interaction with endotoxin
ROYAL SOCIETY (CHARITABLE)
October 2015 - September 2016
Research collaborations
Prof. Yvonne Perie
Structure-activity relationships between liposomes and complement activation
University of Strathclyde
Prof . Rongjun Chen
Development of, immunologically silent, PEG-alternatives
Imperial College London
Prof. Kirill Afonin
Inflammasome recognition of, and response to, nucleic acid-based nanoparticles
UNC Charlotte
Immunocompatibility assessment of, and structural relationships between, nucleic acid nanoparticles.
Dr Rob Vandebriel
Immunotoxicological assessment of nanobiomaterials (REFINE)
RIVM - National Institute for Public Health
Dr Sven Even Borgos
Interindividual variability in Immunotoxicological responses to nanobiomaterials
SINTEF
Dr Joe Vetro
Immunomodulation of macrohage phenotype using nano-polyplexes
University of Nebraska Medical Centre
Dr Joe Vetro
Nucleic acid based nano-therapies for cancer
University of Nebraska Medical Centre
Research partnership to develop, biocompatible, nucleic acid based therapies for drug resistant cancers.
Dr Bryant Nelson
Standardsation of immunotoxicological assessment of nanobiomaterials
National Institutes of Health (NIH and ASTM
Prof. Patricia Murray
Assessment of novel model systems to investigate renal inflammation
Dr Bettina Wilm
Impact of extracellular vesicles on macrophage function and phenotype
Prof, Rachel Williams
Biocompatibility of advanced materials, with nano-topography
Dr Tom McDonald
Development of, immunomodulatory, lipidic nanoparticle delivery systems.