Democracy for electrons?
Spin-coupled theory has been used to investigate the bonding in several hypercoordinate and 'normal
octet' compounds of main group elements. It was found that d basis functions play much the same qualitative
role in hypercoordinate and normal molecules, acting as polarization functions. There are no obvious
demarcations in the energy penalty per bond of excluding such functions. No evidence was found to support
the traditional notions of spndm hybridization. The spin-coupled approach, also
known as the full GVB model, provides a very clear and simple picture of the bonding in all of
the molecules studied. In SF6, for example, the sulfur atom contributes six equivalent, nonorthogonal
spx-like hybrids which delocalize onto the fluorine atoms. Each of these two-centre orbitals
overlaps with a distorted F(2p) function, with the perfect-pairing spin function dominating. The spin-coupled
description of PF5 is entirely analogous, with remarkably little differentiation between axial and equatorial
bonds. A key consideration for all of the hypercoordinate species studied is the polarity of the various
bonds.
We have suggested that less emphasis than hitherto be placed on the 'octet rule' and that the so-called
democracy principle be asserted: any valence electron can participate in chemical bonding if
provided with a sufficient energetic incentive. This idea was pursued for phosphorus and sulfur halides, for
XeF2, and for the (CH5)-, (SiH5)- and (SiF5)- anions. It is argued that there are no significant qualitative
differences between the hypercoordinate nature of first-row, second-row and noble gas atoms in appropriate
chemical environments.
Spin-coupled orbitals in SF6

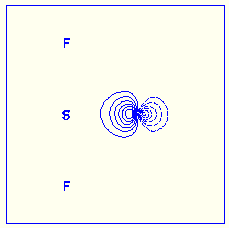
A nonbonding spin-coupled orbital on sulfur in various oxides, fluorides
and oxofluorides





