A new way to synthesise element 116 opens the doorway to even heavier atoms and the elusive “island of stability”
Researchers at Berkeley Lab’s 88-Inch Cyclotron, including members of our Nuclear Physics Cluster, have successfully made superheavy element 116 (livermorium: Lv) using a beam of titanium-50. That milestone sets the team up to attempt making the heaviest element yet: 120. The production of superheavy elements (SHEs), and the investigation of their properties, stands as an important frontier in modern nuclear physics. Their existence was first theorised in the 1950s as the result of stabilisation of very heavy (𝐴 ~ 300), neutron-rich (𝑁 ~ 184) nuclei due to the presence of closed nuclear shells for both protons and neutrons. Today, the concept of an “island of stability” remains an intriguing topic, with its exact position and extent on the Segre chart continuing to be a subject of active pursuit both in theoretical and experimental nuclear physics.
Over the decades, SHEs from 𝑍 = 104 to 118 were discovered using different types of nuclear reactions. Between 2000 and 2016, five new elements were added to the periodic table and over 50 isotopes with 𝑍 = 104–118 were discovered. Since many of these are located near the island of stability, these discoveries have provided crucial insights into the chemistry and physics of SHEs. One of the key focuses of the field is now on the production of new SHEs. Presently, oganesson (Og, 𝑍=118) marks the limit for the production of SHEs using calcium-48 beams. To attempt production of new elements with 𝑍 = 119 or 120 requires a heavier beam, hence the present study involving titanium-50.
This work represents the first published measurement of the production of a superheavy element near the “island of stability” with a beam of titanium-50. It is an essential precursor in the pursuit of searching for new elements beyond 𝑍 = 118 and landing on the shore of the island at the extremes of nuclear mass and charge. A paper has recently been published on this work:
https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.133.172502
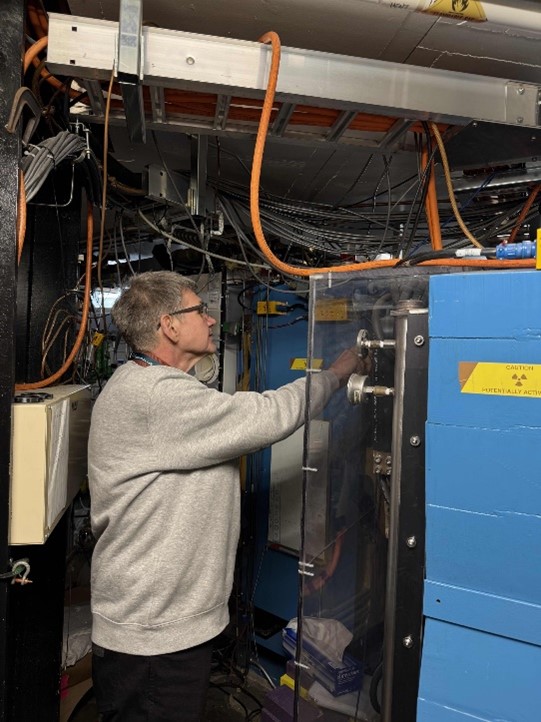
Figure: Rodi Herzberg at the Berkeley Gas-filled Separator used to identify atoms of element 116.