This publication outlines the principles of causality assessment of neurological events following vaccination, in the context of COVID-19 vaccines. We propose categorisation of events into probable, possible and unlikely, where previous evidence is limited. See Table 3 below taken from the publication to be used as a tool.
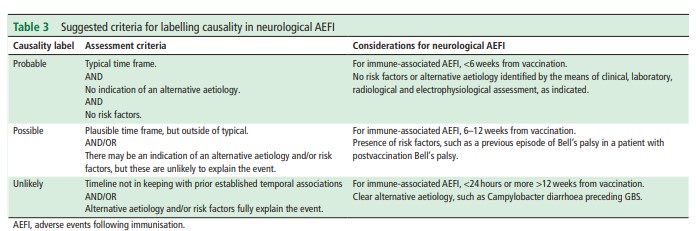
Download a pdf version of Table 3: Suggested criteria for labelling causality in neurological AEFI to zoom in and read the tool more clearly.
This categorisation has been successfully applied in the study of Guilian- Barré syndrome following vaccination in group publication.
Download the full text version of the article 'Considerations for causality assessment of neurological and neuropsychiatric complications of SARS-CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder' published in the Journal of Neurology, Neurosurgery and Psychiatry by clicking on the link to the right.
When to use?
As a companion to the vaccination clinical record forms and in clinical assessment of links between immunisation and neurological events.
Creative Commons License for Causality assessment for Adverse Events Following Immunisation (AEFI)

This CC license means that you are free to use all the files within the resource and are able to share and adapt these files within your organisation.