Invivyd announces positive phase 1/2 clinical data for VYD2311, a monoclonal antibody to be a alternative to COVID-19 vaccination for the broad population

On 3 February 2025, Invivyd, Inc. announced positive data from its ongoing Phase 1/2 clinical trial of VYD2311, Invivyd's novel monoclonal antibody (mAb) candidate designed to be a superior alternative to COVID-19 vaccination for the broad population as frontline protection in a convenient form, as well as to provide a novel, potent, long-acting option for the treatment of COVID-19.
COVID-19 in 2024 caused approximately 59,0001 deaths, 665,0001 hospitalizations, and growing burden of Long COVID over time, despite broadly available and utilized vaccine boosts and small molecule therapy. The initial Phase 1/2 data support VYD2311's potential as a more effective and convenient option to manage this deadly disease.
Invivyd's ongoing randomized, double-blind, Phase 1/2 clinical trial is evaluating the safety and clinical pharmacokinetic (PK) profile of VYD2311 in 40 subjects across multiple routes of administration and dose levels for a single dose, and includes VYD2311 dosed intravenously (IV), intramuscularly (IM), and subcutaneously (SC) in four cohorts of 10 patients each, randomized 8:2 to receive drug or placebo. The Phase 1/2 clinical trial has fully enrolled, and all planned doses have been administered, with only long-term follow-up remaining.
While the Phase 1/2 trial remains blinded, all pooled, blinded adverse events (AEs) identified to date across all arms remain mild to moderate and largely confined to typical injection site reactions or infusion reactions.
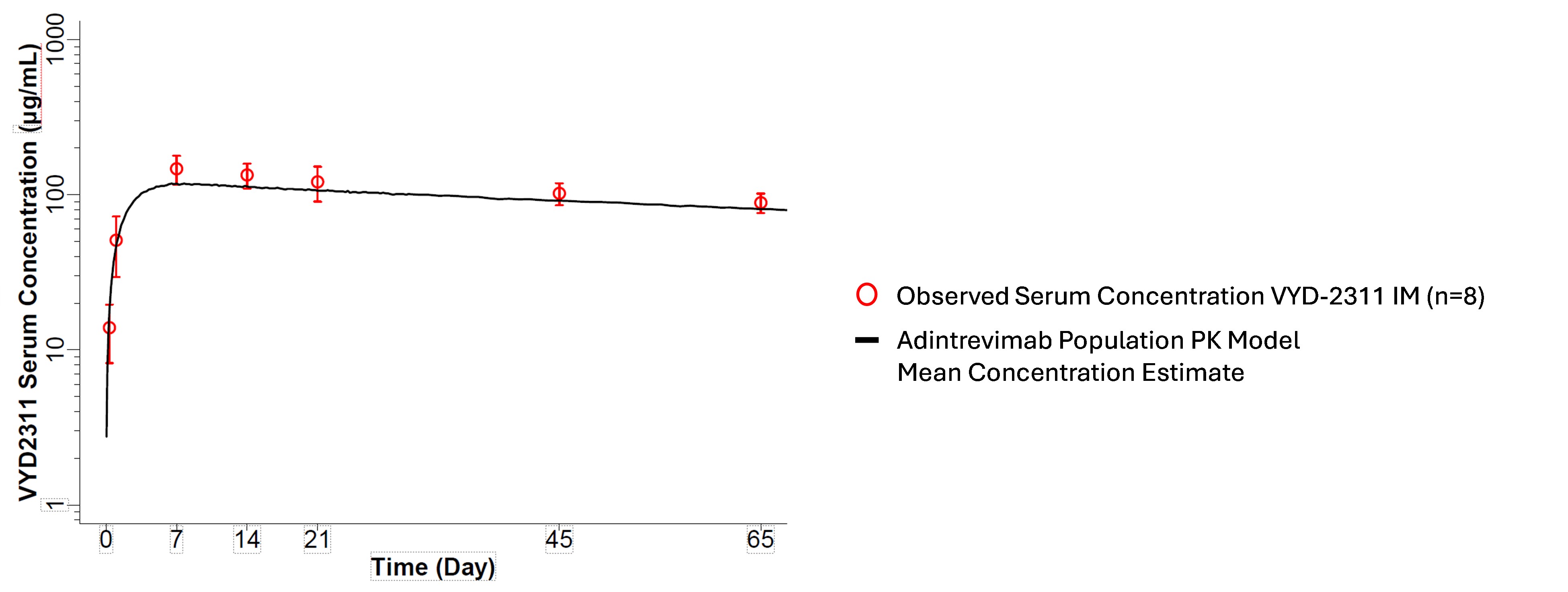
A formal estimate of in vivo half-life is not yet available for VYD2311 given the long apparent half-life thus far. As of Day 65, serum concentrations remain high and represent a potential substantial increase in observed half-life of VYD2311 relative to pemivibart. Analysis for the IM cohort (the most advanced cohort in time) is tracking generally with the PK profile of adintrevimab, a previous Invivyd mAb with an estimated in vivo half-life of 139 days, as depicted in Figure 1 below. PK analysis of VYD2311 intravenous and subcutaneous cohorts at earlier timepoints, at either similar doses subcutaneously or higher doses intravenously, are similarly and encouragingly tracking close to the estimated curves for adintrevimab thus far.
Figure 1: Comparison of VYD2311 Measured Serum Concentration and Modeled Adintrevimab Concentration at Same Timepoints
In vitro neutralization data generated continuously on VYD2311 as part of Invivyd's industrial virology efforts demonstrate an average potency improvement for VYD2311 of approximately 17-fold compared to pemivibart, which is a benefit that is directly translatable into either an equivalent reduction in dose relative to PEMGARDA™ (pemivibart), or a corresponding increase in antiviral activity relative to PEMGARDA at equivalent doses.
Taken together, these VYD2311 safety, PK, and virology data, along with the data from Invivyd's CANOPY Phase 3 clinical trial for pemivibart2 and other prior mAb pre-exposure prophylaxis studies3, predict an attractive clinical protective profile of VYD2311, especially relative to COVID-19 vaccination, including likely highly protective titers of VYD2311 for up to six months or a year or more following a single dose. Shorter dosing intervals (e.g., quarterly or semi-annual) via either IM injection by a healthcare provider or at home via SC self-administration may be attractive for immunocompromised persons or other populations who may benefit from higher serum virus neutralizing antibody (sVNA) titers and, therefore, higher protection, and who also do not wish to interact regularly with healthcare infrastructure to acquire protection from COVID-19. Further, IM administration or higher doses of VYD2311 via IV infusion may be an attractive profile for purposes of delivering maximum possible antiviral activity to patients in need of treating active COVID-19.
We are thrilled with these Phase 1/2 data for VYD2311, which we designed for the profile we are seeing so far: a potent and long-lasting mAb with the potential to protect people with none of the well-understood limitations of COVID-19 vaccination, and which, if brought to market, we believe can meaningfully interrupt disease in a fashion that is difficult or impossible for COVID-19 vaccination to ever achieve. Use of prior mAbs has been constrained by virus evolution, but the science we see now from our platform technology allows us to move game-changing technology like VYD2311 to the center of managing this deadly and pervasive virus. We look forward to collaborating with the U.S. FDA as we work to advance this important medicine to populations at highest risk of COVID-19.
commented Marc Elia, Chairman of the Invivyd Board of Directors.
Last year, while sadly not broadly appreciated, more Americans died of COVID-19 than of breast cancer, and the growth in the long-term damage caused by COVID-19 (Long Covid) continues to mount in this endemic, mass disabling phase of our experience with SARS-CoV-2. Tens of millions of Americans remain at risk despite using serial boosts of vaccines that give short and modest protection from disease. While we are proud of our growing PEMGARDA business serving certain immunocompromised persons, we are seeking to maximize the medical and social impact of Invivyd's transformational science. Deploying a new protective mAb in the future that has the convenience and scalability of current vaccine boosts but with substantially more attractive protection, safety, and durability would be a massive step change in medical value for millions of Americans in need.
said Tim Lee, Chief Commercial Officer of Invivyd.
VYD2311 was discovered via Invivyd technology that performs directed evolution of predicate antibodies toward higher potency against more contemporary SARS-CoV-2 virus lineages and has a highly similar epitope as parent molecule pemivibart and grandparent molecule adintrevimab, which epitope has been genetically and structurally stable over countless virus variants since the emergence of Omicron phylogeny virus.
For more information, read the original press release.
For more news from the world of long-acting therapeutics, sign up to the CELT's LONGEVITY mailing list here for regular updates.