Abstracts: Advances in long-acting therapeutics meeting 2024
Please find below the abstracts for talks and posters at the 2024 Advances in Long-acting Therapeutics Meeting
Oral presentation abstracts
Presented below in running order. Please check back as these are added to.
Day one (7 October)
Formulating stable suspensions for long-acting applications
René Holm
Department of Physics, chemistry and pharmacy, University of Southern Denmark, Campusvej 55, 5230 Odense, Denmark
Patient centricity is widely recognized in drug development, particularly for age-appropriate oral medications, given that most prescribed medications are still conventional tablets or capsules. This concept involves designing medicines that focus on the physiological, physical, psychological, and social needs of individual patients or distinct populations6,10. Patient-centric drug development identifies these needs to create pharmaceutical products with the best overall benefit-to-risk profile for the intended treatment duration. An interesting perspective from the FDA's patient-focused drug development initiative in 2014 highlighted the views of HIV patients on current treatments. Patients valued once-daily and single-pill regimens for adherence but expressed a preference for less frequent dosing, such as long-acting injectables (LAIs) [1;2]. Similar preferences have been noted among schizophrenic patients [3]. Adding to this, analysis of clinical studies comparing oral treatment with LAI based treatment have demonstrated better treatment outcome in the latter case [4], hence the LAI technologies have huge untapped potential.
Aqueous crystalline suspensions are formulations in which the active pharmaceutical ingredient (API) exists in crystalline form with a defined particle size (typically ranging from 0.1 µm to 10 µm) and is dispersed in an aqueous medium [5-7]. This method is a sustained-release strategy for long-acting injectables (LAIs), leveraging the low water solubility and high lipophilicity of the API. The release rate of LAI suspensions is commonly manipulated by altering the particle size distribution of the crystalline API. This principle is explained by the Noyes-Whitney equation [8], which describes the dissolution rate of particles under sink conditions:
where m is the total dissolved mass, Sw is the total exposed surface area of the particles, D is the diffusion coefficient of the solute in the solvent, h is the thickness of the diffusion layer around the particle, Cs is the solubility, and Cb is the concentration of dissolved drug at any given time. According
to the Noyes-Whitney equation, the dissolution rate is proportional to the total surface area of the drug particles, making this the most critical formulation parameter for LAI suspensions.
Excipients in suspension formulations typically include surfactants, stabilizers, tonicity agents, and buffers when needed. Surfactants act as wetting agents, enabling more efficient processing in top-down manufacturing and preventing particle agglomeration in the drug product. Common wetting agents used are non-ionic surfactants like poloxamer-338, polysorbate-20, and polysorbate-80 [9]. Surfactant molecules adsorb on particle surfaces and provide steric hindrance, reducing the likelihood of particle contact. The type and concentration of surfactants and stabilizers depend on the particle size distribution, physicochemical properties of the API, and its tendency to form agglomerates. Although empirical assessments can predict the amount of surfactant needed to cover particle surfaces effectively, formulation screening is essential to identify optimal ranges and ensure long-term stability. In this talk examples on how to obtain stable suspensions through application of a range of formulation and processing techniques will be exemplified and discussed.
References:
1. FDA, 2014; The Voice of the Patient. Human Immunodeficiency Virus (HIV). Patient-Focused Drug Development and HIV Cure Research. https://www.fda.gov/media/88257/download, accessed accessed 06-July-2023
2. Margolis, D. A.; Gonzalez-Garcia, J.; Stellbrink, H. J.; Eron, J. J.; Yazdanpanah, Y,: Podzamczer, D.; Lutz, T.; Angel, J. B.; Richmond, G. J.; Clotet, B.; Gutierrez, F.; Sloan, L.; Clair, M. S.; Murray, M.; Ford, S. L.; Mrus, J.; Patel, P.; Crauwels, H.; Griffith, S. K.; Sutton, K. C.; Dorey, D.; Smith, K. Y.; Williams, P. E.; Spreen, W. R. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017, 390, 1499-1510.
3. Blackwood, C.; Sanga, P.; Nuamah, I.; Keenan, A.; Singh, A.; Mathews, M.; Gopal, S. Patients' preference for long-acting injectable versus oral antipsychotics in schizophrenia: results from the patient-reported medication preference questionnaire. Patient Prefer. Adherence 2020, 14, 1093-1102.
4. Okoli, C. T. C.; Kappi, A.; Wang, T.; Makowski, A.; Cooley, A. T. The effect of long-acting injectable antipsychotic medications compared with oral antipsychotic medications among people with schizophrenia: A systematic review and meta-analysis. Int. J. Ment. Health Nurs. 2022, 31, 469-535.
5. Kipp J. E. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int. J. Pharm. 2004, 284, 109-122.
6. Holm R.; Lee R. W.; Glassco, J.; DiFranco, N.; Bao, Q.; Burgess, D. J.; Lukacova, V.; Alidori, S. Long-acting injectable aqueous suspensions - summary from an AAPS workshop. AAPS J. 2023, 25, 49.
7. Bauer, A.; Berben, P.; Chakravarthi, S. S.; Chattorraj, S.; Garg, A.; Gourdon, B.; Heimbach, T.; Huang, Y.; Morrison, C.; Mundhra, D.; Palaparthy, R.; Saha, P.; Siemons, M.; Shaik, N. A.; Shi, Y.; Shum, S.; Thakral,
N. K.; Urva, S.; Vargo, R.; Koganti, V. R.; Barrett, S. E. Current state and opportunities with long-acting injectables: industry perspectives from the innovation and quality consortium "Long-acting injectables" working group. Pharm. Res. 2023, 40, 1601-1631.
8. Dokoumetzidis, A.; Papadopoulou, V.; Macheras, P. Analysis of dissolution data using modified versions of Noyes-Whitney equation and the Weibull function. Pharm. Res. 2006, 23, 256-261.
9. Alidori, S.; Subramanian, R.; Holm, R. Patient-centric long-acting injectable and implantable platforms – an industrial perspective. Mol. Pharm. 2024, in press.
Peptide-like hydrogels as long-acting injectable platforms for combined HIV/AIDS-contraceptive drug delivery
G. Laverty1, S.M. Coulter1, S. Pentlavalli1, Y. An1, H. Sun1, J.V. Moore1, E.R. Cross1, M. Zbiri2, R. Schweins2
1. Queen’s University Belfast, School of Pharmacy, BT9 7BL, Belfast, N. Ireland.
2. Institut Laue – Langevin, CS 20156, Grenoble, France.
Introduction: There is a need for a convenient and effective long-acting formulation to deliver a combination of HIV/AIDS and contraceptive drugs. Women in the developing world represent are the most significant group for new HIV/AIDS infections.1 Our aim was to develop an injectable in situ forming hydrogel depot for the delivery of antiretroviral (zidovudine, MIV-150, cabotegravir) and contraceptive drugs (etonogestrel) for ≥28 days using peptide-like molecules (D-peptides, peptoid-D-peptides) that form hydrogels in response to phosphatase enzymes present in the subcutaneous space. 2,3 The peptide-like formulation enables drugs to be conjugated covalently forming a readily injectable solution.
Methods: Drug-peptides synthesised using solid-phase synthesis. Mechanical properties characterized using oscillatory rheology. The structure of hydrogel fibre networks studied using small angle neutron scattering (SANS). Cell toxicity assessed using MTS, Live/Dead and LDH assays. Biostability studied using protease Proteinase K. Drug release from hydrogels assessed for 28 days in PBS (pH 7.4) and drug plasma concentrations over 5 weeks in Sprague Dawley rats.
Results: Rheology showed peptides rapidly formed hydrogels within minutes in the presence of phosphatase enzyme. SANS demonstrated peptide gels closely fit model data for flexible cylinder elliptical model. The inclusion of non-native peptoid monomers and D-variant amino acids confers protease resistance, enabling enhanced biostability to be demonstrated in vitro for at least 28 days. For example, burst release from physically encapsulated MIV-150 and etonogestrel combinations was reduced by >40% (first 72 hours) via chemical conjugation of drugs to the same D-peptide gelator (NapffkYG-OH). This system was also able to deliver clinically relevant plasma concentrations of drugs (MIV-150 >4x respective IC90 value and etonogestrel’s mean trough concentration of 0.153 ng/mL (based on Nexplanon’s® data) for up to 42 days in rats.
Conclusions: This work is a proof-of-concept for the development of a long-acting combined injectable in situ forming depot using a peptide hydrogel formulation strategy. Our discrete technology can provide extended contraceptive cover and HIV/AIDS prevention within one product, empowering women to take control of their sexual health and improving adherence to medication.4 The platform also holds immense promise in the treatment and prevention of wider chronic and infectious diseases.
Acknowledgements: Funding provided by EPSRC, MRC, Wellcome Trust, Innovate UK and Invest NI. SANS/QENS at ILL experiment numbers 9-13-972, 8-04-935 and 9-13-1100.
References:
1. Karim,S. et al. Lancet Global Health 2019, 7, e1470-e1471.
2. Coulter, S. M et al. Advanced Healthcare Materials 2023, 12 (18), 2203198. DOI: 10.1002/adhm.202203198.
3. Coulter, S. M et al. Journal of the American Chemical Society 2024. DOI: 10.1021/jacs.4c03751.
4. UNAIDS. Fact sheet-Global HIV & AIDS statistics 2022.
In situ forming implants based on nanogels for long-acting drug delivery
T. McDonald, E. Niezabitowska, D. Gray, E. Gallardo-Toledo, A. Owen, S.P. Rannard
Treating patients with chronic diseases represents an increasing on global healthcare systems in the future. Although chronic disease represents a vast range of conditions, the success of therapeutic treatment for these conditions faces a common challenge: global patient adherence to treatment regimens is typically poor, resulting in increased morbidity and mortality. Therefore, designing systems that can provide long-acting drug release implants may offer a solution to this adherence issue. We have designed a degradable, synergistic dual-stimuli system to form injectable implants that provide long-term drug release. [1-7] This long-acting drug delivery can potentially address the issue of poor adherence and improve patient outcomes for chronic disease.
Our drug delivery system consists of a colloidal assembly of synergistic dual-stimuli responsive nanogels and solid drug nanoparticles. The nanogels respond to the dual-stimuli of body temperature and physiological ionic strength to form a defined colloidal assembly only upon injection into the tissue. The injection of a concentrated dispersion of solid drug nanoparticles with the nanogels results in a nanocomposite drug depot with high drug loading. This talk will cover the development of in situ forming implants based on multi-responsive polymers and the pre-clinical evaluation of these systems.
References:
1. E. Niezabitowska, D. M. Gray, E. Gallardo-Toledo, A. Owen, S. P. Rannard and T. O. McDonald, Understanding the Degradation of Core-Shell Nanogels Using Asymmetrical Flow Field Flow Fractionation, Journal of Functional Biomaterials, 2023, 14, 346.
2. D. M. Gray, A. R. Town, E. Niezabitowska, S. P. Rannard and T. O. McDonald, Dual-responsive degradable core–shell nanogels with tuneable aggregation behaviour, RSC Advances, 2022, 12, 2196–2206.
3. L. Johnson, D. M. Gray, E. Niezabitowska and T. O. McDonald, Multi-stimuli-responsive aggregation of nanoparticles driven by the manipulation of colloidal stability, Nanoscale, 2021, 13, 7879–7896.
4. E. Niezabitowska, A. R. Town, B. Sabagh, M. D. Morales Moctezuma, V. R. Kearns, S. G. Spain, S. P. Rannard and T. O. McDonald, Insights into the internal structures of nanogels using a versatile asymmetric-flow field-flow fractionation method, Nanoscale Advances, 2020, 2, 4713–4721.
5. A. Town, E. Niezabitowska, J. Kavanagh, M. Barrow, V. R. Kearns, E. García-Tuñón and T. O. McDonald, Understanding the Phase and Morphological Behavior of Dispersions of Synergistic Dual-Stimuli-Responsive Poly( N -isopropylacrylamide) Nanogels, The Journal of Physical Chemistry B, 2019, 123, 6303–6313.
6. A. R. Town, J. Taylor, K. Dawson, E. Niezabitowska, N. M. Elbaz, A. Corker, E. Garcia-Tuñón and T. O. McDonald, Tuning HIV drug release from a nanogel-based in situ forming implant by changing nanogel size, Journal of Materials Chemistry B, 2019, 7, 373–383.
7. A. R. Town, M. Giardiello, R. Gurjar, M. Siccardi, M. E. Briggs, R. Akhtar and T. O. McDonald, Dual-stimuli responsive injectable microgel/solid drug nanoparticle nanocomposites for release of poorly soluble drugs, Nanoscale, 2017, 9, 6302–6314.
Preclinical development of long-acting injectable formulations of glecaprevir and pibrentasvir for hepatitis C treatment in low- and middle-income countries
Catherine Unsworth, Andrew B. Dwyer, Alison Savage, Jonathan Massam, James J. Hobson, Cameron Hogarth, Andrew Owen, Steve P. Rannard
Centre of Excellence for Long-acting Therapeutics, University of Liverpool, Liverpool, UK
Hepatitis C is a leading cause of mortality worldwide, and low- and middle-income countries carry a disproportionate burden in terms of case numbers, and the cost of treatment. Although curative therapies are available, lengthy oral dosing regimens contribute to compromised adherence and can be cost-prohibitive. Glecaprevir (GLE) and pibrentasvir (PIB) are direct-acting antivirals (DAAs) which are co-formulated as a fixed dose combination (FDC) and administered orally as three daily tablets for a period of 8-12 weeks. An administration form that could offer longer dosing intervals could improve hepatitis C cure rates.
Solid drug nanoparticle (SDN) generation methodologies have been reported as a suitable route to production of high-concentration particle dispersions of active pharmaceutical ingredients. If such SDN materials are generated with suitable material properties, they could be used to develop parenterally administrable therapies such as long-acting injectables (LAIs). LAI SDN formulations of GLE, PIB and GLE+PIB as an FDC have been developed using emulsion-spray drying, and optimised to enable maximum syringability, the highest mass that can be withdrawn and discharged through a suitably sized syringe and needle. Formulation parameters including mass of excipients within the formulation, and processing parameters including process solvents, influenced behaviour and properties of the SDN powders upon reconstitution. It was also shown that SDN material characteristics could be adjusted in the early stages of formulation development to influence potential administration challenges, such as injection site discomfort. Sustained in vitro release of GLE from a high-concentration SDN dispersion was demonstrated, but the suitability of SDN dispersions to long-acting delivery of GLE, PIB and GLE+PIB under physiological conditions can only be demonstrated through in vivo studies.
Acknowledgements: This work was supported by funding through global health agency Unitaid project LONGEVITY.
Dose linearity studies of a glecaprevir and pibrentasvir long-acting injectable formulation in Sprague Dawley rats
Eduardo Gallardo-Toledo1, 2, Usman Arshad1, 2, Henry Pertinez1, 2, Joanne Sharp1, 2, Joanne Herriott1, 2, Edyta Kijak1, 2, Helen Cox1, 2, Alison C. Savage2, 3, Catherine Unsworth2, 3, Andrew B. Dwyer2, 3, James J. Hobson2, 3, Lee Tatham1, 2, David L. Thomas4, Paul Curley1, 2, Steve Rannard2, 3, Andrew Owen1, 2
1. Department of Pharmacology and Therapeutics, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool, UK.
2. Centre of Excellence in Long-acting Therapeutics (CELT), University of Liverpool, Liverpool, UK.
3. Department of Chemistry, University of Liverpool, Liverpool, UK
4. Department of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Background: Glecaprevir (G) and pibrentasvir (P) is a fixed-dose combination (FDC) approved to treat all six types of hepatitis C. However, patient adherence to oral treatment regimens remains a major challenge with considerably lower efficacy in clinical use than reported in RCTs. Long-acting injectables (LAI) could address poor adherence through long-term exposure of both G and P after a single administration.
Materials and methods: Male Sprague Dawley rats (n = 4 per group) were intramuscularly dosed into both thighs with LAI suspensions of G and P alone, and both drugs in a FDC (GP-FDC, 1:1 ratio) as follows: GP-FDC group (75 mg G + 75 mg P, 150 µL/thigh), G group (150 mg G, 150 µL/thigh), P group (150 mg P, 150 µL/thigh), and GP group (75 mg G, 150 µL/left thigh + 75 mg P, 150 µL/right thigh). A second set of experiments evaluated the effects of different GP-FDC active doses (18.75, 37.5, and 75 mg) on the pharmacokinetics (PK) by changing the dosing volume (0.075, 0.15 and 0.3 mL of GP-FDC at 500 mg/mL) or GP-FDC suspension strength (0.3 mL of GP-FDC at 125, 250, and 500 mg/mL). For all studies, blood samples were collected from the lateral tail vein up to 90 days post dose. G and P concentrations were quantified in plasma using a validated method by LC MS/MS.
Results: GP-FDC showed plasma concentration-time profiles above the reported median human Ctrough for both G and P over the 90 days. However, for single drug-LAI suspensions, plasma concentrations of P were above the human Ctrough over 70 days for both P alone and GP groups, whereas a more rapid drop in plasma concentrations were observed for G in both G and GP groups, after 35 and 28 days, respectively. In the second set of experiments, a linear dose dependent PK was observed with increasing volume, with a proportional increase in the AUC0-tlast for both G and P (G: 106, 220, and 390 μg·h/ml and P: 157, 346, and 513 μg·h/ml for 0.075, 0.15, and 0.3 mL, respectively). Conversely, when dose was titred by GP-FDC suspension strength, a non-dose linear increase in the AUC0-tlast for both G and P was observed (G: 156, 325, 390 μg·h/ml and P: 200, 400, 513 μg·h/ml for 125, 250, and 500 mg/mL). Notwithstanding, both experimental conditions provided appropriate plasma exposures; while the 18.75 mg dose maintained G and P exposure above the human Ctrough for 5 and 11 weeks, respectively, the 37.5 and 75 mg doses maintained plasma exposures above the human Ctrough for both G and P throughout the 90 days.
Conclusions: Plasma exposure of both G and P between GP-FDC and single drug-LAI suspensions suggested that P helps to maintain a longer terminal half-life for G. Moreover, PK data demonstrate a sustained exposure over a period of 90 days for both G and P in rats when novel GP-FDC is administered. Optimisation of drug ratios, as well as GLP toxicology assessments, is required to progress to human clinical trials.
Cell membrane-derived nanoparticles as highly selective brain-targeted drug delivery systems
Christos Tapeinos and Yixuan Yan
University of Manchester
Cell membrane-based nanoparticles (CM-NPs) are promising advancements in targeted drug delivery and cellular uptake. These innovative nanocarriers leverage the natural properties of cell membranes to enhance biocompatibility and specificity towards specific cells and tissues. This study investigates the design and functionality of CM-NPs, focusing on their ability to target various cell types and facilitate efficient internalisation selectively. We assess their performance in in vitro models, demonstrating that CM-NPs can effectively navigate biological barriers and improve therapeutic outcomes. The results highlight the potential of CM-NPs as versatile platforms for targeted drug delivery, offering significant implications for treating various diseases, particularly in challenging environments such as the central nervous system. Future research will aim to optimise CM-NP formulations and validate their efficacy in vivo, paving the way for their clinical application in precision medicine.
Niclosamide exerts its immune modulatory properties primarily through altered glucose homeostasis and subsequent changes in immune cell bioenergetics - towards a rational development for long-acting immunotherapy
Luke R. Wingrave1, 2, Danielle E. Brain1, 2, Micheal C, Munson1, 2, Bethany J, Heaton1, 2, Neill J.Liptrott1, 2
1. Immunocompatibility Group, Department of Pharmacology and Therapeutics, Institute of Systems, Molecular and Integrative Biology, The University of Liverpool, Liverpool, UK
2. Centre of Excellence for Long-acting Therapeutics (CELT), Department of Pharmacology and Therapeutics, Institute of Systems, Molecular and Integrative Biology, The University of Liverpool,
Glucose uptake, via glucose transporters (GLUTs), and subsequent glucose metabolism is essential for cell function. Immune cells especially require rapid glucose uptake to support critical processes and activities such as rapid proliferation and increased biomolecule synthesis upon immune stimulation through the generation of ATP and needed biomass [1]. Under stimulated conditions, immune cells primarily switch to the less efficient but more rapid glycolysis pathway to satisfy the increased ATP demand [2]. Previous research within the group has shown that smallmolecule drugs can impact immune cell function through GLUT interactions, thereby inhibiting responses to prototypical stimulants [3, 4]. Initially, this work was to support investigation of the impact of niclosamide on immune responses, to treat SARS-CoV-2 infection. However, in the community, niclosamide and other mitochondrial uncouplers, are increasingly being assessed for applications in non-communicable diseases such as cancer and autoimmune disease [5-7]. The potential efficacy of niclosamide in other conditions such as neurodegenerative diseases like amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS) may benefit from the immunomodulatory effects [8, 9]. In the current study, it was observed that glucose accumulation and lactate secretion was greater in cells following 24-hour niclosamide exposure compared to untreated cells, potentially due to induced GLUT4 expression in exposed cells. Compensatory glycolysis rates were lower with drug exposure, and higher concentrations further inhibited glycolysis regardless of agonist pre-activation. Cytokine and chemokine secretion following niclosamide exposure was dependent on agonist pre-activation, indicating the immunomodulatory effects of niclosamide are influenced by the pre-existing activation status of the immune cells. Conditions such as ALS and MS currently suffer from a sparsity of effective treatment options, and those presently available may require frequent dosing. Repurposing drugs such as niclosamide and reformulating them into long-acting injectables may significantly reduce the time burden felt by those suffering these conditions and drastically improve quality of life. The data presented here supports the immunomodulatory potential of niclosamide and demonstrates possible reasons for conflicting reports on the activity of niclosamide in the literature. Additionally, our findings provide a platform for evaluating and repurposing licensed drugs for diseases that would benefit from modulation of immune responses.
References:
1. Marchingo, J.M. and D.A. Cantrell, Protein synthesis, degradation, and energy metabolism in T cell immunity. Cellular & Molecular
Immunology, 2022. 19(3): p. 303-315.
2. Palmer, C.S., et al., Glucose Metabolism Regulates T Cell Activation, Differentiation, and Functions. Frontiers in Immunology,
2015. 6.
3. Heaton, B.J., et al., Exposure of human immune cells, to the antiretrovirals efavirenz and lopinavir, leads to lower glucose uptake
and altered bioenergetic cell profiles through interactions with SLC2A1. Biomedicine & Pharmacotherapy, 2022. 150: p. 112999.
4. Brain, D., et al., Drug delivery systems as immunomodulators for therapy of infectious disease: Relevance to COVID-19. Adv Drug
Deliv Rev, 2021. 178: p. 113848.
5. Kumar, R., et al., Mitochondrial uncoupling reveals a novel therapeutic opportunity for p53-defective cancers. Nature
Communications, 2018. 9(1): p. 3931.
6. Haase, S. and R.A. Linker, Inflammation in multiple sclerosis. Ther Adv Neurol Disord, 2021. 14: p. 17562864211007687.
7. Staats, K.A., et al., Blood-based biomarkers of inflammation in amyotrophic lateral sclerosis. Molecular Neurodegeneration, 2022.
17(1): p. 11.
8. Liang, L., et al., Inhibitory effects of niclosamide on inflammation and migration of fibroblast-like synoviocytes from patients with
rheumatoid arthritis. Inflamm Res, 2015. 64(3-4): p. 225-33.
9. Al-Gareeb, A., F. Gorial, and A. Mahmood, The Anti-Rheumatoid Activity of Niclosamide in Collagen-Induced Arthritis in Rats. Arch
Rheumatol, 2019. 34(4): p. 426-433.
Day two (8 October)
Microneedle systems for administration of long-acting therapeutics
Ryan Donnelly
Long-acting (LA) therapeutics are becoming increasingly important in management and prevention of chronic diseases. They overcome many of the issues associated with long-term treatments, enhancing compliance and reducing side-effects. However, the majority of LA treatments require injection or implantation, both painful, invasive procedures, that require healthcare professional intervention and generate potentially-hazardous sharps waste. These issues cause particular problems in developing countries, where cold chain transport and storage of liquid preparations is also problematic.
Microneedle arrays are minimally-invasive systems that painlessly and without drawing blood penetrate the skin’s stratum corneum barrier to enable deposition of LA therapeutics in the viable skin layers for subsequent release and absorption.
In this presentation design and evaluation of a range of microneedle systems for administration of LA therapeutics will be discussed from a scientific and translational viewpoint, with a focus on delivery of drugs for HIV, malaria, schizophrenia and cardiovascular disease.
Addressing unresolved challenges in cancer therapy using localised drug delivery
Maria Marlow
University of Nottingham
Dr Marlow’s research is inspired by her industrial career. The motivation for her research is to address unmet clinical needs, using localised drug delivery to specific tissues. Her research is focused on the use of device and formulation combinations including hydrogels, nanoparticles, and microneedles. The lecture will describe data for targeted formulation and device approaches to the CNS and skin to treat brain tumours (glioblastoma) and skin cancer (basal cell carcinoma) using pectin hydrogels, polymer coated nanocrystals and polymer microneedles.
More information about these projects and associated publications can be found at:
Find Dr Marlow's publications list here.
Acknowledgements:
Brain tumours (glioblastoma): Paula Muresan Phoebe McCrorie, Fiona Smith, Catherine Vasey, Vincenzo Taresco, David J. Scurr, Stefanie Kern, Stuart Smith, Pavel Gershkovich, Ruman Rahman
Skin cancer (basal cell carcinoma): Akmal Sabri, David Scurr, Joel Segal, Fiona Smith, Lindy Durrant, Victoria Brentville
Delivering a step-change in the characterisation of complex medicines
Karen Alvey
University of Nottingham
The increasing complexity of modern medicines, driven by novel therapeutic targets and active ingredients, poses significant challenges in formulation and delivery. For example, recent mRNA-based therapeutic products, have required a multi-component, multi-layered lipid formulation to ensure its stability and therapeutic effectiveness.
Traditional analytical methods in pharmaceutical development are well-established, but as medicines grow more complex, more advanced technologies are needed to fully understand and control critical quality attributes and process parameters. Many current techniques lack the necessary chemical specificity, spatial resolution, or sample compatibility to provide the molecular-level insights required to link formulation structure with function. This presentation will review traditional approaches for characterising pharmaceutical particles and highlight recent advances in electron microscopy and mass spectrometry. These innovations provide new, detailed insights into formulation structure and composition, enabling a deeper understanding of complex medicines and supporting more precise manufacturing processes.
Dissolving microarray patches for malaria chemoprophylaxis: Mapping solid drug nanoparticle incorporation and long-acting release in vivo
Sam Morris, Elliot Croft, Andrew Owen, Steve Rannard and Helen Cauldbeck
Introduction: Atovaquone, a first-line chemoprophylactic antimalarial, is currently administered as a daily oral dosing regimen for travellers to malaria endemic areas. Transdermal drug delivery (TDD) is an attractive alternative to repetitious oral delivery, circumventing common patient adherence issues such as pill fatigue. Nanoformulation of non-water soluble ATQ allows the generation of an aqueous dispersion, subsequent encapsulation within polymeric dissolvable microarray patches (DMAPs) and facilitates efficient TDD of ATQ, which may provide a long-acting, patient acceptable, alternative to current ATQ dosing regimens.
Methods: ATQ solid drug nanoparticles (SDNs) were prepared and formulated into polyvinylpyrrolidone (PVP) DMAPs at 3 different concentrations via solvent casting micromoulding.1,2 DMAP physicochemical properties were evaluated via uniaxial insertion force measurement into ex vivo porcine skin. ATQ distribution within DMAPs was measured using scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDXS), X-ray microtomography (XCT) and radiometric sectioning. Release of ATQ from DMAPs was measured in vitro, ex vivo utilising porcine ear skin, and in vivo using a Sprague-Dawley rodent model.
Results: Uniform PVP DMAPs containing ATQ SDNs were prepared with considerable tip-loading of ATQ (Fig. 1). SDNs (315 – 380 nm) were stable to DMAP formulation and dissolution – maintaining their nanosize on redispersion.
.jpg)
Fig. 1 i) XCT reconstruction of ATQ SDN-loaded DMAP. ii) XCT cross-sectional density analysis with radiometric quantification of 3H-ATQ tip sequestration. iii) SEM (A) and EDXS (B) analysis of broken needle tip showing ATQ-rich crystallites.
Complete DMAP insertion into ex vivo porcine skin was confirmed via uniaxial compression analysis with insertion forces generable by ‘thumb force’ (≤ 20 N).
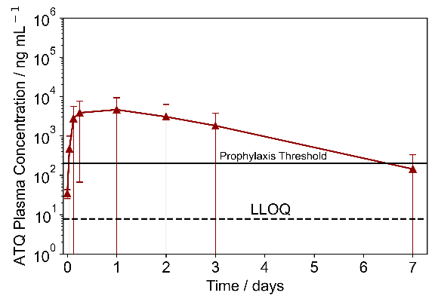
Fig. 2 Pharmacokinetic profile of ATQ released from DMAPs.
Concentration-dependent ATQ release was observed in vitro from DMAPs with larger ATQ loadings. Ex vivo and in vivo analyses indicated long-acting ATQ release from DMAPs; ATQ plasma concentrations exceeded the prophylactic threshold (200 ng mL-1) for approximately 7 days (Fig. 2) and an allometric t1/2 suggestive of sustained release in human subjects.
Conclusions: Nanoformulation of ATQ into SDNs facilitated dispersion in aqueous polymeric DMAP substrates. DMAPs loaded with ATQ SDNs were generated and displayed characteristics for long-acting TDD of antimalarial chemoprophylaxis.
References:
1 R. P. Bakshi, L. M. Tatham, A. C. Savage, A. K. Tripathi, G. Mlambo, M. M. Ippolito, E. Nenortas, S. P. Rannard, A. Owen and T. A. Shapiro, Nat. Commun., 2018, 9, 1–8.
2. I. K. Ramöller, E. McAlister, A. Bogan, A. S. Cordeiro and R. F. Donnelly, Micromachines, 2020, 11, 1–12.
Learning Outcome 1: Methods for qualitative and quantitative analysis of ATQ distribution within DMAPs.
Learning Outcome 2: Interpretation of in vitro, ex vivo and in vivo data indicating long-acting delivery from DMAPs.
Acknowledgements: This work was supported by funding through global health agency Unitaid project LONGEVITY (2020-38-LONGEVITY)
Biography: Postdoctoral researcher within the Department of Chemistry at the University of Liverpool. Specialises in radiometric techniques for the development and assessment of TDD devices.
Long Acting Injectables- The Future of Affordable Malaria Prevention
Laxman Cherkupally
Medicines for Malaria Venture
Malaria remains a significant global health challenge, necessitating innovative approaches to prevention and treatment. Long-acting injectable (LAI) dosage forms have emerged as a promising solution for malaria chemoprevention, offering sustained drug release and improved patient adherence. It is imperative to know the critical importance of LAIs in malaria prevention, highlighting their potential to transform the landscape of malaria control.
The development of aqueous suspensions as LAIs presents unique challenges and benefits. On one hand, these formulations ensure prolonged drug action, reducing the frequency of administration and enhancing patient compliance. On the other hand, the formulation and stability of aqueous suspensions pose significant technical hurdles, including particle size control, reconstitution, and syringeability/injectability. Despite these challenges, the benefits of LAIs in providing consistent therapeutic levels and minimizing the risk of resistance development are substantial.
The journey of developing MMV055 and MMV371 as long acting injectables exemplifies these challenges. The co-formulation of MMV055 and MMV371 further required meticulous optimization of formulation and manufacturing process and involved several intricate steps to ensure the stability and quality of the drug product. These long-acting injectable dosage forms hold great promise for malaria chemoprevention, offering a viable solution to improve patient adherence and reduce malaria incidence. However, the journey has just begun, and more time and effort are needed to refine these formulations and address the remaining challenges to ensure their successful market introduction.
LAPaL - An Innovative Tool for Tracking Global Advances in Long-acting Therapeutics Clinical Development and Regulatory Approval.
M.P. Ryan1, P. Venkatasubramanian1, S. Morin2, A. Larbi2, C. Flexner3, A. Owen1, L. Gaayeb2, A. Olagunju1
1. Centre of Excellence for Long-acting Therapeutics, University of Liverpool, Liverpool, UK
2. Medicines Patent Pool, Geneva, Switzerland
3. The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Background: Given the pace of development in the field, tracking latest innovations and developments in long-acting therapeutics is becoming increasingly important, but challenging for researchers, funders, advocacy groups, regulators and policy makers. Here we present the Long-Acting Therapeutics Patents and Licences Database (LAPaL), an open, access-oriented platform containing expertly curated information on long-acting therapeutics and technology platforms, including their global clinical development and regulatory approval status.
Methods: LAPaL is the first and only collaborative database tracking the clinical development and regulatory status of long-acting therapeutics. Launched in 2021, it contains detailed and current information regarding their potential applications, formulation properties, manufacturing requirements, scale-up prospects and intellectual property. Priority is given to therapeutics with current or potential application for indications of global health importance, including in the fields of HIV, malaria, viral hepatitis and tuberculosis. Information is collected from publicly accessible sources, including peer-reviewed journal articles, patents, conference proceedings and commercial press releases. Clinical trial information is sourced directly from trial sponsors websites and online registries. Regulatory approval data is compiled from national formularies, drug registries, and approved drug databases maintained by individual countries. Data validation of published technology entries is enhanced through verification by the innovator. Users can dynamically filter or search available technologies and compounds by therapeutic area, technology type and route of administration, in addition to differentiating between small molecules and biotherapeutics. LAPaL also features interactive dashboards of clinical trials and regulatory data, providing a one-stop shop for users seeking an overview of the long-acting therapeutics landscape.
Results: A total 18 drug technologies and 24 long-acting compounds have been published as of July 2024. Primary routes of administration for curated technologies include subcutaneous (72%), intramuscular (27%), and topical (27%), while 44% support multiple routes. Supported dosing frequencies include monthly (n = 17), weekly (n = 14), biyearly (n = 7), yearly (n = 4), and other intervals (n = 2). Clinical development stages of the curated compounds include Preclinical (29%), Phase I (4%), Phase I/II (4%), Phase II (33%), Phase III (21%) and Marketed (8%). For HIV therapy, four compounds were approved in low- and middle-income countries based on the global regulatory map. These include cabotegravir (approved n = 17; submitted n = 7), cabotegravir & rilpivirine (approved n = 6), dapivirine (approved n = 11; submitted n = 1), lenacapavir (approved n = 1). These compounds are indicated for pre-exposure prophylaxis (PrEP) (n = 2) and treatment (n = 3) of HIV. Eligibility for HIV PrEP and treatment trials was highest for adults at 98% of studies and lowest for children at 6%. Older adults are eligible for 35% of PrEP and 80% of treatment trials, compared with 12% and 6% adolescents, respectively.
Conclusion: LAPaL provides users with access to latest innovations, supports access and encourages collaboration. Alongside additional long-acting compounds and technologies, LAPaL will continue to receive iterative updates and new features will be implemented to cater to the needs of stakeholders.
Poster presentation abstracts
Presented below in alphabetical order of presenter surname. Please check back as these are added to.
Development of a USP-4 IVIVC Method for Cabotegravir and Rilpivirine as case studies for Long-Acting Injectables
Emad Anaam, Henry Pertinez, Eduardo Gallardo-Toledo, Lee Tatham, Anthony Valentijn, Paul Curley, Joanne Sharp, Andrew Owen
Centre of Excellence for Long-acting Therapeutics (CELT), Department of Pharmacology and Therapeutics, Institute of Systems and Molecular Biology, University of Liverpool, Liverpool, UK.
Background: Long-acting injectable (LAI) antivirals offer a promising approach to improving adherence and efficacy in the treatment of HIV. Establishing a reliable in vitro-in vivo correlation (IVIVC) is critical for predicting the in vivo performance of these formulations. This study aims to develop a USP apparatus 4 (USP-4) IVIVC methodology specifically for LAI antivirals, using Cabotegravir (CAB) and Rilpivirine (RPV) as model compounds.
Material and Methods: CAB and RPV formulations were subjected to in vitro release testing using the USP-4 apparatus with dose release from a float-a-lyzer dialysis device. Method optimisation investigated adjustment of parameters such as temperature, dialysis membrane MWCO, and flow rate, Tween 20 excipient concentration and pH of the circulating buffer. Cumulative release profiles were sampled and analysed over a period of 30 days and fitted with a biexponential mathematical model to allow extrapolation to estimate longer duration release. For in vivo analysis, clinical LAI pharmacokinetic (PK) data for CAB and RPV were obtained from literature, and in vivo release profiles derived through deconvolution using intravenous (IV) bolus disposition impulse responses estimated for both drugs via PBPK scaling due to lack of clinical IV data. IVIVC predictions and comparisons were made both by convolution of the in vitro release with IV disposition for direct comparison with the in vivo PK exposure profile, and by Levy-plot correlations of in vitro and in vivo cumulative release.
Results: Both CAB and RPV formulations show a biphasic, biexponential release profile in-vitro. The initial release phase is shorter for CAB (~1 week) than for RPV (~2 weeks). Extrapolation of the in-vitro release predicts 50% and 20% in-vitro release for CAB and RPV respectively after durations to match in-vivo profiles. Predictions from convolution of in-vitro release with estimated IV disposition generally overpredict the observed PK profiles for both CAB and RPV (with a period of mid-profile underprediction for CAB). Considering release alone, lower cumulative release is shown in-vitro on the in-vivo profile duration, reflected in the shape of the Levy correlation plots.
Conclusions: Initial results from developing a USP-4 methodology for LAI formulations and establishing an IVIVC with clinical LAI PK exposures indicate some potential for predicting in vivo PK, with some demonstration of a degree of correlation between in vitro and in vivo data. However, the rate and extent of in-vitro release is generally slower over the full in vivo time course. The presented methodology has potential utility in ranking and comparing formulations according to in-vitro release rate, but further optimisation is warranted. The observed discrepancies between in vitro and in vivo data highlight the need for further adjustments to the experimental setup. Optimisation of experimental parameters is ongoing to expand the library of LAI formulations being investigated and identify trends across LAI products with a broader range of physicochemical properties and release mechanisms, to assess generalisability of the methodology.
Investigating the Pharmacokinetic Effect of Recombinant Human Hyaluronidase PH20 on the Release Profile of Subcutaneously Administered Long-Acting Injectable Suspension in Rats
Prithi Balarasa1 and René Holm11
1. Department of Physics, Chemistry, and Pharmacy, University of Southern Denmark
Suspensions are a frequently used strategy for long-acting injectables with commercial products releasing the active ingredient for up to six months. These suspensions are injected intramuscularly in volumes up to 5mL [1]. However, technical limitations on the drug dose in suspensions and other long-acting injectable formulation strategies constrain the variety of molecules suitable within this platform.
The subcutaneous tissue comprises the intricate extracellular matrix (ECM). The enzyme Recombinant Human Hyaluronidase PH20 (rHuPH20) fluidizes the ECM, allowing for larger injection volumes and effective subcutaneous delivery of multiple commercial products in the biological space [2] . Therefore, rHuPH20 should be applicable for administering a larger volume of a long-acting injectable suspension, expanding the range of molecules that could be considered for use and extending the release duration of the formulations.
In this study, we investigate how rHuPH20 affects the pharmacokinetic behavior of a commercially available subcutaneous long-acting injectable suspension using in vivo release data from rats to determine the impact of rHuPH20 on the compound's pharmacokinetic profile.
References:
1. Jindal AB, Bhide AR, Salave S, Rana D, Benival D. Long-acting parenteral drug delivery systems for the treatment of chronic diseases. Adv Drug Deliv Rev. 2023 Jul;198:114862. doi: 10.1016/j.addr.2023.114862. Epub 2023 May 7. PMID: 37160247.
2. Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007 Jul;4(4):427-40. doi: 10.1517/17425247.4.4.427. PMID: 17683255.
Assessment of cell phenotype and gene expression changes, following repeat exposure to the NRTIs FTC, 3TC and long acting polymer linear poly(FTC) – relevance to subcutaneous administration of long-acting therapeutics
Danielle Brain1,2, Faye Hern2,3, Anika Shakhil2,3, Chung Liu2,3, Steve Rannard2,3, Andrew Owen2 and Neill Liptrott1,2
1. Immunocompatibility Group, Department of Pharmacology and Therapeutics, Institute of Systems and Molecular Biology, University of Liverpool, Liverpool, UK.
2.Centre of Excellence for Long-acting Therapeutics (CELT), Department of Pharmacology and Therapeutics, Institute of Systems and Molecular Biology, University of Liverpool, Liverpool, UK.
3. Department of Chemistry, School of Physical Sciences, University of Liverpool, Liverpool, UK.
HIV treatment requires chronic administration of antiretrovirals to suppress viral replication, with no current cure (Ruelas & Greene, 2013). Due to the lack of “forgiveness” in current antiretroviral regimens, in terms of viral breakthrough, long-acting antiretroviral medication can help to improve adherence to medication, resulting in better treatment outcomes (Chandiwana et al., 2021). Currently, the nucleoside reverse-transcriptase inhibitors (NRTIs), emtricitabine (FTC) and lamivudine (3TC), are being explored for use in long-acting delivery. A key question in this type of delivery is, with long-acting antiretrovirals, does repeated, and long-term, exposure to these drugs alter the functional capacity of human immune cells. Linear poly(FTC) is a long-acting polymeric prodrug of FTC, which is designed to be delivered subcutaneously as an implant that releases FTC slowly over time (Shakil et al., 2022). As such, it is important to assess its immunocompatibility with relevant immune cell types from the subcutaneous space. MUTZ-3 cells represent a useful model with which to study these interactions (Groell, Kalia, Jordan & Borchard, 2018).
The MUTZ-3 (human dendritic cell (DC) line) were cultured in either standard media or media containing either FTC (1.8 μg/mL), 3TC (2 μg/mL) or linear poly(FTC) (20 μg/mL) for 7 weeks, passaged twice-weekly, followed by an assessment of cellular phenotype, at week 7. Intracellular reactive oxygen species (ROS) were measured using CellROXTM Green Reagent, intracellular reduced glutathione was measured using ThiolTrackerTM Violet Dye and mitochondrial membrane potential (MMP) was assessed using JC-1 reagent (InvitrogenTM). Positive controls, specific to the cell type, were included in the evaluation, cells were treated with either LPS or Resiquimod (R848). Expression of a number of cell-surface markers in each cell line was assessed via multi-parametric flow cytometry. To related to possible subcutaneous administration of long-acting antiretrovirals, the MUTZ-3 cell line was assessed for changes in gene expression using the Nanostring™ metabolic pathways panel nCounter assay for differences between untreated cells and the cells treated with R848.
Intracellular ROS levels were significantly lower (18% and 21% lower respectively) in the FTC- and 3TC-cultured cells, than the untreated cells (p<0.01) (Figure 1a). However, linear poly(FTC)-cultured cells had significantly higher ROS levels, 19% higher than the untreated (p<0.01). FTC- and linear poly(FTC)-cultured MUTZ-3 cells displayed a significantly higher MMP, 29% and 33% higher than the untreated cells (p<0.05) (Figure 1c).
When compared to the untreated cells, the linear poly(FTC)-cultured cells showed a 262% significantly higher CD209 expression (p<0.05), the R848 treated linear poly(FTC)-cultured cells also showed a significantly higher expression of CD209 when comapred to the R848 treated cells, 56% higher (p<0.05) (Figure 1a). Linear poly(FTC)-cultured cells showed a 61% significantly lower expression of HLA-DR, when compared with the untreated cells (p<0.05) (Figure 1e). When compared to the R848 treated cells, the linear poly(FTC)-cultured R848 treated cells had a 76% significantly lower expression of HLA-DR (p<0.05) (Figure 1e). The linear poly(FTC)- cultured R848 treated cells had a 118% significantly higher CD274 expression (p<0.01) (Figure 1f). In the untreated-, 3TC- and linear poly(FTC)-cultured cells treated with R848 CD274 expression was also higher than the untreated counterparts, with 310% (p<0.01), 196% (p<0.05), and 516% (p<0.01) higher expression respectively (Figure 1f).
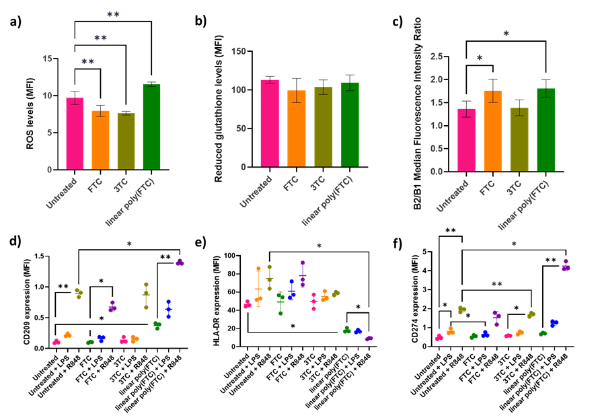
Figure 1: MUTZ-3 cells exposed to FTC and 3TC for 7 weeks and subsequent phenotypic assessment. a) Intracellular ROS, n=4, mean ± SD. b) Intracellular reduced glutathione, n=4, mean ± SD. c) MMP, n=4, mean ± SD. d) CD209 marker expression, n=3 ± SD. e) HLA-DR marker expression. f) CD274 marker expression, n=3 ± SD. P <0.0001 = ****, P< = ***, P <0.01 = ** and P <0.05 = *.
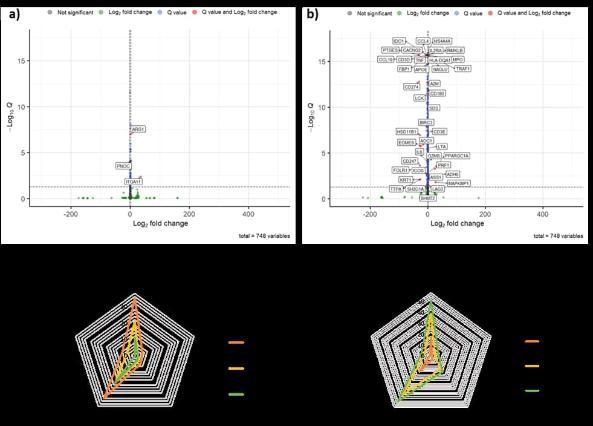
Figure 2: MUTZ-3 cells exposed to FTC and 3TC for 7 weeks and subsequent gene expression assessment. a) Significant gene expression changes when FTC-cultured R848 treated cells were compared to R848 treated cells (q<0.05). b) Significant gene expression changes when linear poly(FTC)-cultured R848 treated cells were compared to R848 treated cells (q<0.05). c) Number of genes within the metabolic pathways panel themes that had a higher expression. d) Number of genes within the metabolic pathways panel themes that had a lower expression.
Only 3 genes showed significantly altered gene expression when the FTC-cultured cells treated with R848 were compared to the R848 treated cells (Figure 2a). Many genes showed altered expression when linear poly(FTC)-cultured cells treated with R848 were compared to the R848 treated cells (Figure 2b). Of note significantly reduced gene expression of CCL4, TNF and CCL19 was seen in the linear poly(FTC)-cultured R848 treated cells compared to the R848 treated cells. Interestingly the CD274 gene expression contradicted what was seen in the marker expression results.
When compared to the untreated cells, the FTC-cultured cells had a greater profile of genes with higher expression and these tended to be within two main areas, Biosynthesis and anabolic pathways or Signalling effecting cellular metabolism (Figure 2c). 3TC then had a lower profile and followed by linear poly(FTC) and still in the same key areas (Figure 2c). Interestingly however when looking at genes that had lower expression, linear poly(FTC)-cultured cells had the largest profile of genes with lower expression and again in the same two themes, 3TC-cultured cells then had the next most changes followed by the FTC-cultured cells and again both within the same two themes (Figure 2d).
Responses to linear poly(FTC) in the MUTZ-3 cell line were marker specific, this is interesting as it indicates there is some activation, but also some inhibition of marker responses as a result of the cell sensing linear poly(FTC). Reduced expression of CCL4, TNF and CCL19 in the linear poly(FTC)-cultured R848 treated cells could suggest reduced activation of these cells. FTC exposure results in a greater profile of genes with higher expression, whereas linear poly(FTC) exposure results in a greater profile of genes with lower expression, this suggests there is a complex relationship between the repeat exposure of these three treatments and the effect on the cells, it is important to next understand the phenotype more clearly as the mRNA levels don’t necessarily translate into increased protein levels, as was seen for CD274. Metabolic processes of these cultured cells should be explored further to better understand the consequences. Previously efavirenz and lopinavir have led to lower glucose uptake and caused bioenergetic modifications to cells, it would be useful to explore the methods used to determine if FTC, 3TC and linear poly(FTC) also alter these profiles (Heaton et al., 2022). These results have, potential, consequences for the development of future long-acting implants as they show possible effects of repeat exposure to antiretrovirals that may be used in such preparations and polymers of these antiretrovirals, and highlight the requirement for early assessment of biocompatibility, as highlighted elsewhere (Su et al., 2020).
References
Chandiwana NC, Serenata CM, Owen A, Rannard S, Pérez Casas C, Scott C, et al. (2021). Impact of long-acting therapies on the global HIV epidemic. AIDS 35.
Groell F, Kalia YN, Jordan O, & Borchard G (2018). Hydrogels in three-dimensional dendritic cell (MUTZ-3) culture as a scaffold to mimic human immuno competent subcutaneous tissue. Int J Pharm 544: 297-303.
Heaton BJ, Jensen RL, Line J, David CAW, Brain DE, Chadwick AE, et al. (2022). Exposure of human immune cells, to the antiretrovirals efavirenz and lopinavir, leads to lower glucose uptake and altered bioenergetic cell profiles through interactions with SLC2A1. Biomed Pharmacother 150: 112999.
Ruelas Debbie S, & Greene Warner C (2013). An Integrated Overview of HIV-1 Latency. Cell 155: 519-529.
Shakil A, Hern FY, Liu C, Temburnikar K, Chambon P, Liptrott N, et al. (2022). Linear and branched polymer prodrugs of the water-soluble nucleoside reverse-transcriptase inhibitor emtricitabine as structural materials for long-acting implants. Journal of Materials Chemistry B 10: 4395-4404.
Su JT, Simpson SM, Sung S, Tfaily EB, Veazey R, Marzinke M, et al. (2020). A Subcutaneous Implant of Tenofovir Alafenamide Fumarate Causes Local Inflammation and Tissue Necrosis in Rabbits and Macaques. Antimicrobial Agents and Chemotherapy 64: e01893-01819.
Harnessing Immunomodulatory Approaches for Targeted Eradication of Glioblastoma: Microneedles for Local Imiquimod Delivery
Yasar Hamed, David Scurr, Pavel Gershkovich and Maria Marlow,
School of Pharmacy, University of Nottingham, Nottingham NG7 2RD, United Kingdom
Background: Glioblastoma (GBM) is a treatment-resistant malignant primary brain tumour that poses a considerable challenge to patients and healthcare professionals. It is one of the most prevalent and resistant tumours of the central nervous system (CNS) and an extremely aggressive form of cancer. This type of tumour severely reduces patients’ quality of life and prognosis, with less than 5% of patients surviving beyond five years and 90% of cases experiencing recurrence post-resection even with the current treatment options (1) . This calls for innovative strategies to enhance traditional therapy options. Immunotherapies stimulate the immune system's response to fight cancer cells and their microenvironment. By focusing on this approach, we can enhance the efficacy of cancer treatments by modulating the body's immune system. In addition, local drug delivery can overcome the BBB, increasing drug delivery at the application site. Recently, we have demonstrated that we can delivery small molecule drugs locally to brain tissue using adhesive microneedle patches(2). Hence, herein we will use these dissolvable microneedles patches loaded with imiquimod to be anchored within the resection cavity following the surgical excision of a tumour, achieving local delivery of high imiquimod concentrations. Delivering imiquimod locally aims to target residual cancer cells, reduce recurrence risk, minimise toxic side effects, and ultimately improve survival rates and quality of life for GBM patients. Additionally, imiquimod's potency is valuable in treating anatomically complex regions where surgical intervention is not feasible.
Methods: Imiquimod-loaded microneedles were moulded using a two-step casting method. Three different polymers —PVPVA, PVA, and poly(N-acryloylmorpholine)(pNAM) — were used to fabricate the needle layer loaded with imiquimod. NaCMC/glycerol was used as a backing layer. Profilometry, optical microscopy, and environmental scanning electron microscopy were used to measure the microneedles' dimensions. The fracture force of the microneedles was measured using a texture analyser. Microneedles were applied ex vivo to one hemisphere of rat brains and HPLC was used to detect imiquimod in multiple layers. Imiquimod concentrations in ex vivo rat brain layers were measured starting from the patch application site, with each layer being 1 mm thick.
Results and discussion: Microscopic analysis of the microneedle patches revealed obelisk-shaped micro projections, matching the expected mould dimensions. The fracture force test showed that all polymers are suitable for brain application. All polymers exhibited a fracture force exceeding that needed to penetrate the skin (0.098 N/needle), which is harder than brain tissue. The PVPVA-based needles loaded with imiquimod had the lowest fracture force (0.241 ± 0.01), while PVA needle layers had the highest (1.020 ± 0.08). These microneedle patches with the CMC backing layer adhered firmly to brain tissue upon application, conforming to its shape.
HPLC methodology was then established where the lower limit of quantification (LLOQ) of imiquimod was found to be 20 ng/ml and the method was found to be linear (r² = 0.997). After PVPVA microneedle application, HPLC analysis indicated the highest imiquimod concentrations in the three outermost brain layers, with values of 113657 ng/g, 67915 ng/g, and 494 ng/g respectively. The concentration significantly decreased with depth, showing minimal levels in the deeper layers: 22 ng/g in both layers 4 and 5, 15 ng/g in layer 6, 5.8 ng/g in layer 7, and 3 ng/g in layer 8.
Conclusions: Our project has demonstrated the successful manufacture of microneedles loaded with imiquimod. After application, the microneedles adhered consistently to ex vivo rat brains, resulting in the diffusion of imiquimod in the brain. This highlights the potential of our microneedle patches for targeted imiquimod delivery to the brain to prevent GBM recurrence.
References:
1. R. Stupp et al., Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England journal of medicine 352, 987-996 (2005).
2. P. Muresan et al., Development of nanoparticle loaded microneedles for drug delivery to a brain tumour resection site. European Journal of Pharmaceutics and Biopharmaceutics 182, 53-61 (2023).
Discovering the Optimal Stabilizer for Pretomanid to Treat Tuberculosis
Mads Hansen1, Nadina Zulbeari1, René Holm1
1. Department of Physics, Chemistry, and Pharmacy, University of Southern Denmark, Odense, Denmark
Introduction: Every day Tuberculous (TB) claims thousands of lives [1]. The development of Pretomanid was initiated in response to the urgent need for new antibacterial drugs against multidrug-resistant tuberculosis (MDR-TB). LAIs have showed to be beneficial for patient groups with long and curial need for medication. Therefore, LAIs seems to be an excellent formulation proposal to treat Tuberculosis (TB), which has a medication treatment of a period of 4-6 month. Aqueous nano- or microsuspensions is known to be thermodynamic unstable due to Ostwald ripening. As the particle size defines the release duration from aqueous suspensions after administration it is one of the key formulation parameters to define when formulating a stable suspension, i.e. selecting of the right stabilizer for the system. [2]
Aim: The aim of the study was to investigate Pretomanid’s ability to be formulated as a physical stable suspension using different polymer or surfactant. Additionally, the study aimed to assess its potential as a LAI application for the treatment of tuberculosis.
Method: The technique used was wet media milling, by dual centrifuge in a DeltaVita 1. The stability of the suspensions was measured by laser diffraction using a Mastersizer 3000 over a period of 4 and 24 weeks, when stored under accelerated conditions at 40 °C. 1 mL of a 3% (m/v) solution of a chosen stabilizer (10 different) and 1000 mg yttrium stabilized zirconium oxide beads with 100 mg of API was placed in a 2 mL microtube and milled at 1500 rpm in 90 minutes, with the cooling system set to 0° C.
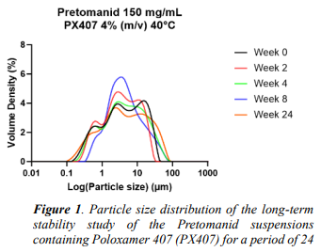
Results: The full stabilizer screening identified Poloxamer 407 as a suitable stabilizer candidate for further study (data not shown). The long-term stability study supported these findings, with only a minor shift of the particle size distribution (see Figure 1). A bead screening was performed to investigate the compound's ability to be milled to the desired particle sizes profile. It revealed that the use of different bead sizes had a very low impact on the compound. Resulting in a relatively high D50 value of 2.02 µm for the smallest bead size investigated with relatively small variations as a function of bead size applied (see table 1). This suggested that Pretomanid have poor brittle properties.
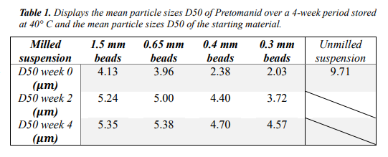
Conclusion: The present study demonstrated that poloxamer 407 was a promising stabilizer candidate for the TB compound Pretomanid. The steric stabilization provided by Poloxamer 407 resulted in a relative physical stable suspension over a 24 weeks’ period. Additionally, it was observed that Pretomanid exhibited poor grindability and brittle properties.
ACKNOWLEDGEMENT
This publication is based on research funded in part by the Bill & Melinda Gates Foundation. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation.
References:
1. WHO Global Tuberculosis Report 2023. Last visited 08-08-24 https://www.who.int/teams/global-tuberculosis-programme/tbreports/global-tuberculosis-report-2023/tb-disease-burden/1-1-tb-incidence
2. Holm, R., Lee, R. W., Glassco, J., DiFranco, N., Bao, Q., Burgess, D. J., Lukacova, V., & Alidori, S. (2023). LongActing Injectable Aqueous Suspensions-Summary From an AAPS Workshop. The AAPS journal, 25(3), 49. https://doi.org/10.1208/s12248-023-00811-8
The effect of polymer type in the in vitro release profile of a BCS Class III drug in formulations of in situ forming implants.
Clarice Sombra de Medeiros1, René Holm1
1. Department of Physics, Chemistry, and Pharmacy, University of Southern Denmark, Odense, Denmark
Introduction: In situ forming implants (ISFI) are long-acting injectable formulations based on biodegradable polymers and water miscible organic solvents. ISFIs undergo solidification due to the solvent exchange and phase separation at the site of injection [1]. The swelling tendencies, solvent efflux and the overall duration of the drug release depend on the type of polymer used in the ISFI formulation [2]. Previous studies have reported more prolonged release profiles with less pronounced initial burst release in ISFI formulations with 1:2 polymer to solvent ratio, demonstrating that it is possible to modulate the drug release in ISFIs by manipulating the formulation composition [3].
Aim: To investigate the effect of polymer type, including molecular weight, L/G ratio, and end-caping on the in vitro release profiles of a BCS Class III drug ISFI prepared with N-methyl pyrrolidone (NMP).
Method: Different ISFI formulations were prepared at a fixed polymer: NMP ratio (1:2 w/w) containing a BCS Class III drug. The amount of drug released was quantified using an Agilent 1100 HPLC system with quaternary pump and diode-array detector. The stationary phase was a Kinetex C18 column (150 mm x 4.6 mm, 5µm). The injection volume was set to 10µL with a flow rate of 1.0 mL/min and mobile phase of water + 0.1% formic acid : acetonitrile, 80:20 v/v. The eluent was monitored at 258 nm. The ISFIs were prepared by mixing the API with NMP at the maximum saturated concentration (123.1± 6.6 mg/mL) and left under rotation overnight. The polymer was added, and the mixture was left under rotation overnight again. The in vitro release studies were conducted in a water bath shaker at 100 rpm and 37oC. 30 uL of the ISFI were injected in 40 mL of phosphate buffer (10 mM) and, at predetermined timepoints, 1 mL of media was extracted for quantification and replaced with fresh media. The entire media volume (40 mL) was replaced for fresh media once a week.
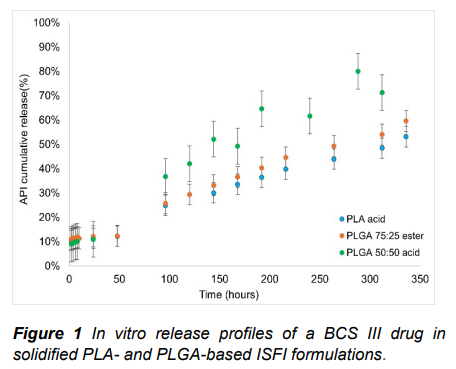
Results: Three different ISFIs formulations containing polymers of low Mw (i.v. = 0.2 dL/g), NMP and API at fixed concentrations were tested in vitro. The initial burst release of the ISFIs did not seem to be affected by the polymer type. However, PLGA 50:50 with uncapped groups presented the fastest release profile, while PLA with uncapped end groups and PLGA 75:25 with esterified end groups
presented similar prolonged release profiles.
Conclusion: The polymer type did not seem to have a significant effect on the initial burst release of the API, however, it can modulate the overall duration of the release. The overall slower release profile of the PLA-based ISFI might be explained by the enhanced hydrophilicity of the uncapped end groups, which increase the waterinflux, reducing the solvent efflux and the drug release.
References
1. Shi, Y. et al. Acta Pharm. Sin. B (2021) 11 (8): 2396-2415.
2. Wang, X & Burgess, D. J. Adv Drug Rev (2021) 178, 113912
3. Kim, M. et al. Nat. Commun. (2022) 13 (1):4455
Nanoprecipitation of niclosamide and in vivo demonstration of long-acting delivery
Catherine Unsworth1, James J. Hobson1, Alison Savage1, Andrew B. Dwyer1,2, Jonathan Massam1, Usman Arshad1, Henry Pertinez1, Helen Box1, Lee Tatham1, Rajith KR Rajoli1, Megan Neary1, Joanne Sharp1, Anthony Valentijn1, Christopher David1, Paul Curley1, Neill J. Liptrott1, Tom O. McDonald1,2, Andrew Owen1 and Steve P. Rannard1,2
1. Centre of Excellence in Long-acting Therapeutics (CELT), University of Liverpool;
2. Materials Innovation Factory, University of Liverpool.
Niclosamide (NCL) is a cheap, broad-spectrum anthelmintic agent with reported antiviral, antibacterial and immunomodulatory properties. However, it is practically insoluble in water, resulting in very low bioavailability after oral dosing, and the current regimen is unsuited to provision of high systemic concentrations, limiting its utility in repurposing. Solid drug nanoparticle (SDN) technologies offer the opportunity to create nanoparticles which can be dispersed in aqueous media at high concentrations, suitable for injectable administration.
We have demonstrated scalable SDN formation of NCL through nanoprecipitation in the presence of biocompatible excipients, followed by spray drying to produce a semicrystalline material. SDN size properties of the redispersed SDNs were characterised by dynamic light scattering (Dz 789 nm, polydispersity index 0.365). The concentration of NCL which could be passed through a syringe and needle of a gauge suitable for injection was assessed. 1H NMR (DMF-d7) was used to confirm the concentration of NCL in SDN powder as 60 wt% relative to excipients.
Suitability of SDN powders for terminal sterilisation through gamma irradiation was investigated. Material characterisation after accelerated storage stability trials showed no distinguishable degradation of NCL post-sterilisation as determined by HPLC analysis, at each of three irradiation doses (15, 25 and 35 kGy), and indicated long-term stability of the powder. The long-acting potential of the NCL SDN formulation was assessed in a pre-clinical study using Sprague Dawley rats. The pharmacokinetic (PK) profile was determined after intramuscular injection at 50, 100 and 200 mg/kg and demonstrated sustained NCL plasma concentrations over 28 days. However, a high initial release (Cmax) was observed.
These in vivo results demonstrate that SDN powders can provide long-acting delivery of NCL, and the powder stability results indicate that the material is suitably robust for translation towards early clinical studies. Improvements to the formulation and delivery method to control the high, initial release of NCL upon injection could increase the period over which NCL is at therapeutic concentrations and reduce the likelihood of any potential side effects. Further studies are investigating the suitability of dispersed NCL SDNs for other parenteral administration routes.
We gratefully acknowledge financial support from the Royal Society of Chemistry collaborations grant, which has enabled this event to be free from registrations costs for delegates. Thanks also go to Selleck for sponsoring the Poster prizes and Marama Labs for event sponsorship.