Clinical trials
We conduct clinical trials from Phase I to Phase IV across all disease areas. We lead both investigator-led studies and collaborative trials with academic and industry partners.
Our clinical researchers have specific interests in the following areas:
- HIV treatment and prevention
- Perinatal pharmacology and drug safety in breastfeeding
- Optimising TB therapy
- Early-phase evaluation of experimental SARS-CoV-2 antivirals
- Treatments for neglected tropical diseases.
Our trials are carried out within the NIHR Liverpool Clinical Research Facility at the Royal Liverpool University Hospital, one of only two MHRA-accredited, first-into-human facilities in the UK with NHS support. This state-of-the-art, 26-bed unit offers inpatient care and advanced research capabilities, including laboratory processing and research bronchoscopies.
To support our clinical trials, we have the Liverpool Good Clinical Practice Laboratory Facility for immunoassays, proteomic and genomic analysis, and a GCP-accredited Bioanalytical Facility equipped with advanced LC-MS technology. These facilities enable us to measure drug concentrations across various biological matrices and integrate this data into population pharmacokinetic models to enhance the understanding of drug behaviour.
We offer a unique environment for clinical trials, combining scientific excellence with a deep understanding of disease biology, especially in molecular pharmacology, infectious diseases, tropical medicine, cardiovascular disease, oncology, and neuropsychiatry. Our experienced team manages each project from concept development through to regulatory submission, trial conduct, and data analysis, ensuring that every study meets the highest standards.
Collaborate with us
We welcome collaborations from academic partners and industry. Our centre is outward-looking, adopting a ‘can do’ approach to new challenges and recognising the need to meet each compound where it is to take it to where it needs to go.
For more information, please visit our Collaborate page.

The NIHR Liverpool Clinical Research Facility has specific experience and expertise in running the highest risk type of trials and those at the earliest stages like first-in-human studies.
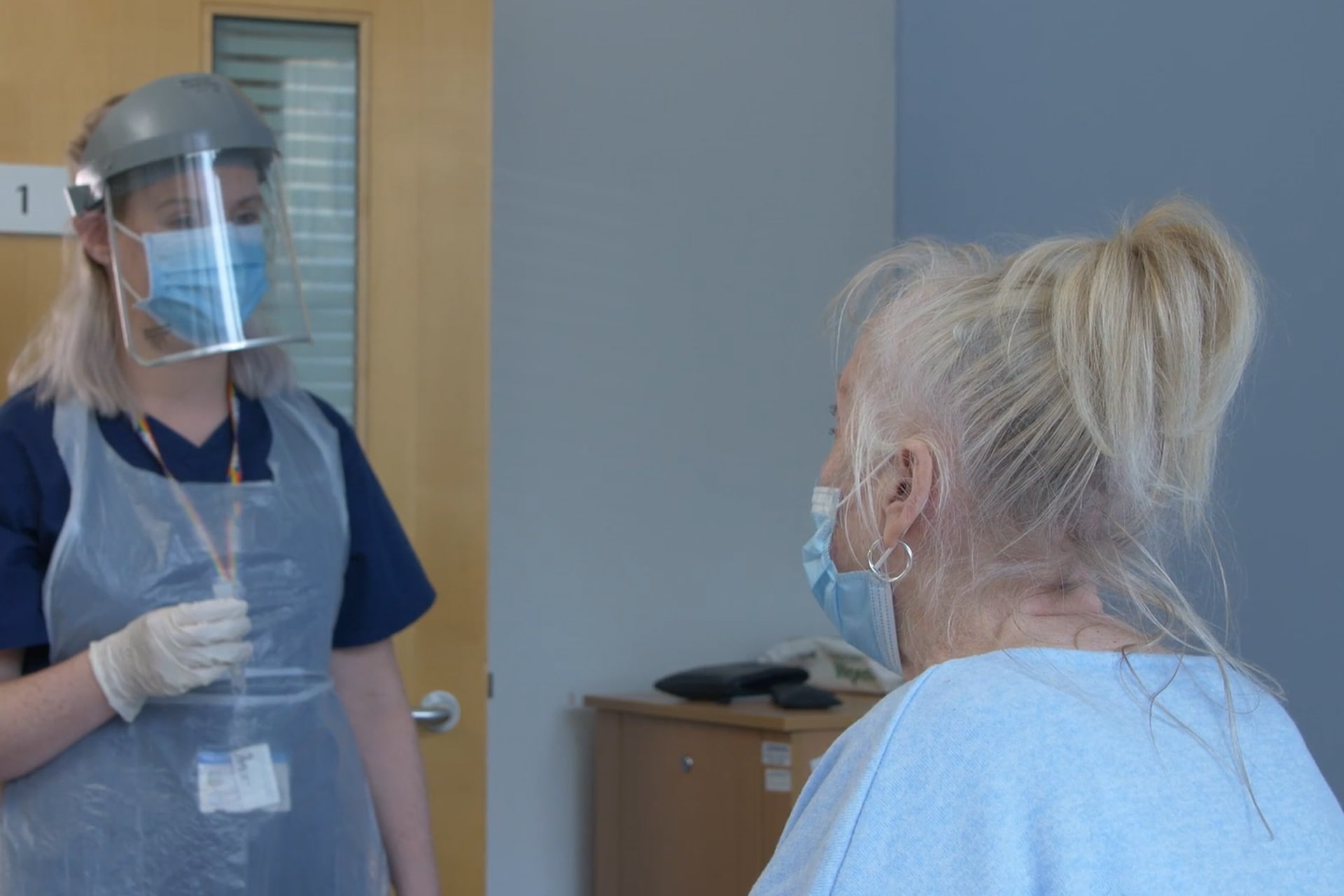
Spotlight: The AGILE Clinical Trial Platform
Creating a new type of clinical trial platform in the wake of the COVID-19 pandemic, and a new way of doing drug development.